(C) 2011 Lenka Moravcová. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Two species of the genus Fallopia (Fallopia sachalinensis, Fallopia japonica, Polygonaceae) native to Asia, and their hybrid (Fallopia ×bohemica), belong to the most noxious plant invaders in Europe. They impact highly on invaded plant communities, resulting in extremely poor native species richness. The low number of native species in invaded communities points to the possible existence of mechanisms suppressing their germination. In this study we assessed, under laboratory conditions, whether there are phytotoxic effects of the three Fallopia congeners on seed germination of three target species: two native species commonly growing in habitats that are often invaded by Fallopia taxa (Urtica dioica, Calamagrostis epigejos), and Lepidium sativum, a species commonly used in allelopathic bioassays as a control. Since Fallopia taxa form dense stands with high cover, we included varying light conditions as an additional factor, to simulate the effects of shading by leaf canopy on germination. The effects of aqueous extracts (2.5%, 5.0%, and 0% as a control) from dry leaves and rhizomes of the Fallopia congeners on germination of the target species were thus studied under two light regimes, simulating full daylight (white light) and light filtered through canopy (green light), and in dark as a control regime. Rhizome extracts did not affect germination. Light treatments yielded inconclusive results, indicating that poor germination and establishment of species in invaded stands is unlikely to be caused by shading alone. However, we found a pronounced phytotoxic effect of leaf extracts of Fallopia taxa, more so at 5.0% than 2.5% extract concentration. Fallopia sachalinensis exerted the largest negative effect on the germination of Urtica dioica, Fallopia ×bohemica on that of Calamagrostis epigejos, and Fallopia japonica had invariably the lowest inhibitory effect on all test species. The weak phytotoxic effect of Fallopia japonica corresponds to the results of previous studies that found this species to be generally a weaker competitor than its two congeners. Although these results do not necessarily provide direct evidence for allelopathic effects in the field, we demonstrate the potential phytotoxic effect of invasive Fallopia taxa on the germination of native species. This suggests that allelopathy may play a role in the impact of Fallopia invasion on species diversity of invaded communities.
Allelopathy, canopy shading, leaf and rhizome extracts, light regimes, phytotoxicity, plant invasions, Reynoutria
Recent research on biological invasions increasingly focuses on different types of impacts of invasive species (
The genus Fallopia is native to Asia and several of the taxa are invasive in Europe (
The ecological understanding of Fallopia invasion and impact is still rather poor; it is usually attributed to a high growth rate (
The extremely low diversity of Fallopia-dominated communities may also be associated with the high cover of Fallopia canopy as it has been repeatedly reported that shading by a dense canopy inhibits germination of some plant species (e.g.
To obtain the first insight into the possible role of phytotoxic compounds and shading in reducing diversity of native species present in Fallopia-invaded stands, we assessed the effects of these factors on germination of co-occurring native species under laboratory conditions. By using extracts from above-ground and below-ground biomass of two Fallopia species and their hybrid, we determined (i) whether the chemical compounds inhibit the germination of two target species, a competitively strong native herb (Urtica dioica) andagrass (Calamagrostis epigejos), which are often dominant species prior to Fallopia invasion. Further, we aimed at assessing (ii) differences in potential phytotoxic action of particular Fallopia taxa that are known to differ in their invasiveness, (iii) differences in potential phytotoxic action of leaves and rhizomes, and (iv) explore how these effects are affected by simulated shading.
Material and methods Target speciesUrtica dioica L. is a perennial forb widely distributed in temperate regions (
Calamagrostis epigejos L. (Roth), a rhizomatous perennial grass of Eurasian distribution (
Besides the two native European taxa, an annual forb Lepidium sativum L., native to SW Asia and NE Africa (
Fruits of target species were collected in Prague, near the Modřanský brook valley (Urtica dioica) and along the road from Modřany to Cholupice village (Calamagrostis epigejos) in September 1999. Seeds of Lepidium sativum were obtained from a seed supplier. Fruits of Calamagrostis epigejos and seeds of Lepidium sativum were stored in paper bags in the dark at room temperature. Fruits of Urtica dioica were cold-stratified at 6 °C for four months before the start of the experiment.
Fresh rhizomes of the three Fallopia taxa were collected in February 1999 in Průhonice near Prague (49°59'41"N, 14°33'56"E). Fresh leaves, without any evident exterior sign of fungal infection, were collected at the same locality in July 1998 and dried at a room temperature.
Extract preparationAqueous extracts from dry leaves. Maximum annual dry biomass produced by Fallopia
in the study area is 228 g/m2 (P. Pyšek et al., unpublished data).
This value was used to set the upper limit of concentrations of
phytotoxic compounds used in the experiment. Leaf biomass produced in
the field was expressed per area of 75 mm Petri dish used for
germination and volume of water it contained (8 ml). This calculation
yielded 126 g of dry leaves per litre, i.e. c. 12.5% solution. However,
since chemicals are released from leaf tissues gradually, we applied
lower concentrations and used 25 and 50 g of dry leaves per litre,
respectively, to produce 2.5% and 5% solutions, which is well within
the concentration range of allelopathic studies (e.g.
Aqueous extracts from rhizomes. Extracts were prepared from fresh washed rhizome cuttings and soaked in 100 ml of distilled water for one week at 20 °C, with occasional aeration to prevent anaerobic conditions. The solutions were filtered through filter paper. Amount of rhizome tissues used corresponded to 10, 20 and 30 g per litre, respectively, yielding 1%, 2% and 3% solution. Microbial degradation of the solution was not observed.
Light regimes appliedThree light regimes were used. Light bulbs and
fluorescent tubes provided a white light of 5000 L (105 mmol/cm2/s–1),
simulating daylight in stands not covered by Fallopia
species. Light filtered through canopy (green light) was simulated by
using a single layer of green plastic reducing the red : far-red ratio
by 69% and the photon flux density by 56% of white light levels (
Germination was tested in growth chambers. Seeds of Lepidium sativum (25 seeds), Urtica dioica (50) and Calamagrostis epigejos (50) were placed on two layers of filter paper in 75 mm diameter Petri-dishes and filled with 8 ml of leaf/rhizome extract or distilled water. Filter paper was used as a sterile medium. In the white- and green-light treatments, the evaporated water was replenished where it dropped below a standard mark. Dark treatments wrapped in aluminium foil could not be filled-up with water during the course of the experiment, but the foil prevented any loss by evaporation. At the end of the experiment we ensured that the petri-dishes did not dry out.
All germination experiments were performed at fluctuating temperature of 25/10 °C (14 h light period/10 h-dark period). Germinating seeds were counted three times a week. Dark treatments were assessed at the end of the experiment, which was terminated after 21 days.
Experimental designThe extracts were first used to conduct preliminary tests on the Lepidium sativum seed bioassay. Germination was assessed under controlled conditions (distilled water), two concentrations of dry leaf extract (2.5 and 5%) and three concentrations of fresh rhizome extract (1%, 2% and 3%) of Fallopia sachalinensis, Fallopia japonica and Fallopia ×bohemica with white light. Five replicates were used in each treatment. Since none of the three concentrations of rhizome extracts of the three Fallopia taxa had significant effect on germination of Lepidium sativum, and seeds of the other two target species germinated up to 100% in most taxon/rhizome extract combinations, fresh rhizome extracts were not used in the main experiment as Lepidium sativum is considered to be very sensitive to the allelopathic action.
In the main experiment, we used two concentrations (2.5% and 5%) of leaf extracts from Fallopia sachalinensis, Fallopia japonica and Fallopia ×bohemica, and a control (distilled water = 0%). These combinations were assessed under three light regimes, to test germination response of the three target species, giving the total of 315 Petri dishes (6 taxon/concentration combinations + 1 control × 3 receiver species × 3 light regimes × 5 replicates).
Statistical analysisData on the proportion of germinated seeds were
angular transformed, and evaluated by fixed effect factorial ANOVAs.
The preliminary test was done with a one-way ANOVA, using control and
different concentrations of aqueousextracts from leaves and rhizomes of Fallopia sachalinensis, Fallopia japonica and Fallopia ×bohemica
as levels of a factor. Differences between control and each extract
were assessed by t-tests. The main experiment was evaluated by three-way
ANOVA, using the target species (Lepidium sativum, Urtica dioica and Calamagrostis epigejos),
the light intensity (white, green, dark) and the concentration of
aqueousextracts (control, 2.5 and 5% dry leaf extract from Fallopia sachalinensis, Fallopia japonica and Fallopia ×bohemica)
as factors. A priorinotions on differences among the concentrations
within each target species and light intensity were tested by orthogonal
contrasts (e.g.
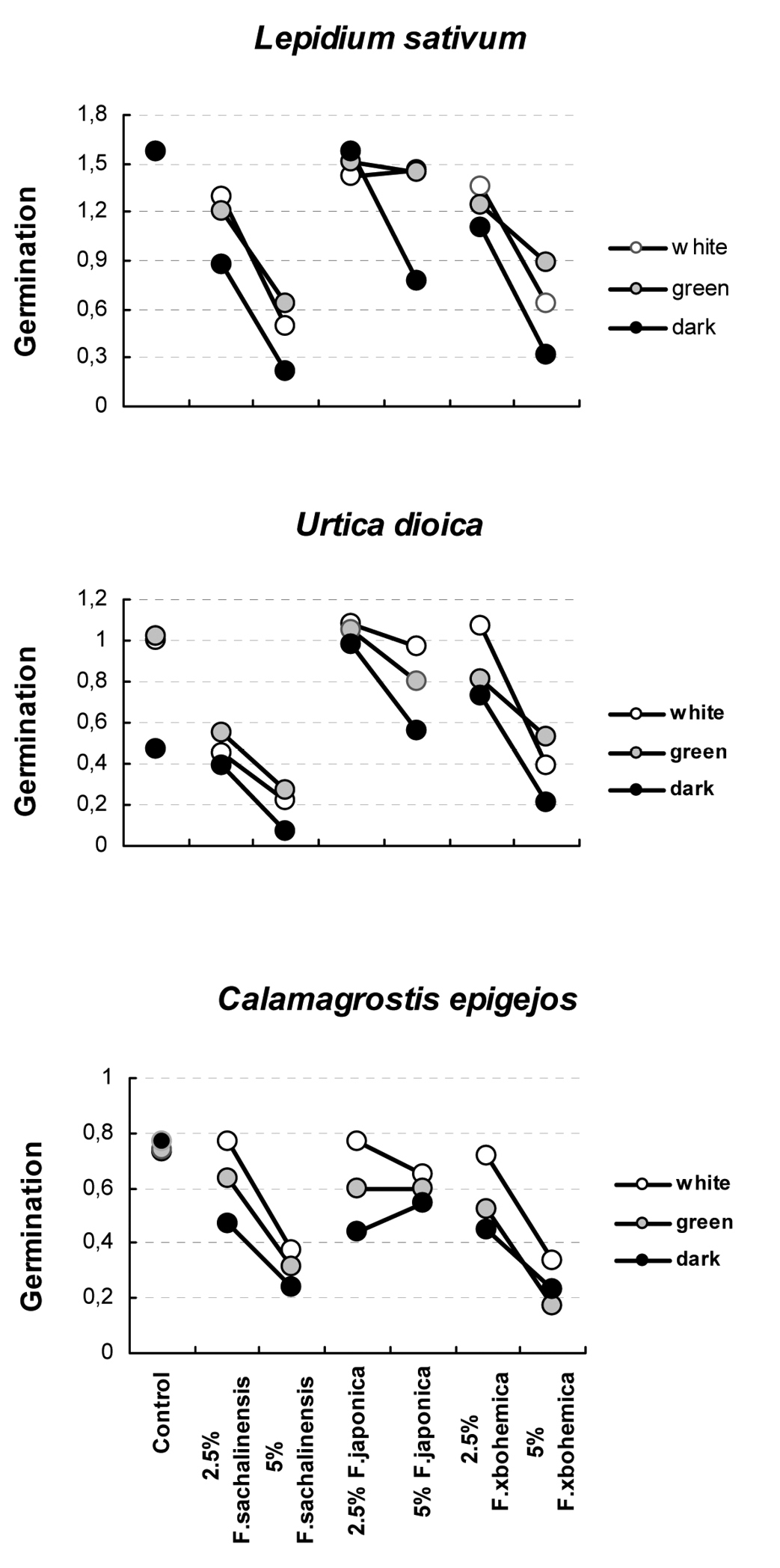
The germination of target species varied under the combination of different treatments, from 8.0 to 100.0% in Lepidium sativum, from 1.2 to 75.2% in Urtica dioica and from 6.4 to 97.3% in Calamagrostis epigejos (Table 1). Germination was highly significantly affected by the interaction between target species, light regime and leaf extract concentration from the three Fallopia taxa (Table 2), and by the interactions between light and leaf extracts within each of the three target species (Table 3 and Fig. 1). In Lepidium sativum, the 5% leaf extract from Fallopia japonica markedly decreased germination in dark, but not in green and white light; the germination then increased on 2.5% extract from Fallopia japonica in dark, but decreased in both light regimes. In Urtica dioica, the decrease in germination between control and 2.5% extract from Fallopia sachalinensis was much steeper in light regimes than in dark. In Calamagrostis epigejos, the germination in white light on 2.5% extract from Fallopia japonica increased compared to control with no extract; on 5% extract from Fallopia japonica germination increased in dark but decrease in both light regimes (Figure 1).
Percentage germination of target species under different combination of light treatments (WL – white light; GL – green light; dark), taxon (FS – Fallopia sachalinensis; FJ – Fallopia japonica; FB – Fallopia ×bohemica) and concentration of the solution (%).
| Target species | Urtica dioica | Calamagrostis epigejos | Lepidium sativum | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment | WL | GL | Dark | WL | GL | Dark | WL | GL | Dark |
| Control | 69.6 | 70.8 | 20.8 | 89.6 | 90.4 | 97.3 | 100.0 | 100.0 | 100.0 |
| 2.5% FS | 19.2 | 28.0 | 14.8 | 96.0 | 71.2 | 42.4 | 92.0 | 86.4 | 58.4 |
| 5% FS | 6.8 | 8.0 | 1.2 | 29.6 | 20.0 | 12.0 | 23.2 | 35.2 | 8.0 |
| 2.5% FJ | 73.2 | 75.2 | 68.4 | 96.0 | 64.0 | 38.0 | 96 | 98.4 | 100 |
| 5% FJ | 67.6 | 51.2 | 28.8 | 72.8 | 20.0 | 53.6 | 96.8 | 96.0 | 48.8 |
| 2.5% FB | 71.6 | 52.4 | 45.2 | 86.4 | 50.4 | 37.6 | 93.6 | 88.0 | 78.4 |
| 5% FB | 15.2 | 28.0 | 4.8 | 21.6 | 6.4 | 11.2 | 36.0 | 59.2 | 12.0 |
Three-way factorial ANOVA of angular-transformed proportions of germinated seeds for the target species (Lepidium sativum, Urtica dioica and Calamagrostis epigejos), light intensity (white, green, dark) and concentration of aqueousextracts (control, 2.5% and 5% dry leaf extract from Fallopia sachalinensis, Fallopia japonica and Fallopia ×bohemica). The main effects (target species, light and concentration) and first-order interactions (target species) × (concentration), (target species) × (light), and (light) × (concentration), were not tested because the second-order interaction (target species) × (concentration) × (light) was statistically significant.
| Source of variation | SS | df | MS | F |
|---|---|---|---|---|
| Target species | 19.25 | 2 | 9.625 | |
| Light | 3.18 | 2 | 1.59 | |
| Concentration | 23.17 | 6 | 3.862 | |
| (Target species) × (Concentration) | 4.57 | 12 | 0.381 | |
| (Target species) × (Light) | 0.52 | 4 | 0.130 | |
| (Light) × (Concentration) | 0.80 | 12 | 0.0669 | |
| (Target species) × (Concentration) × (Light) | 2.01 | 24 | 0.08387 | 4.94 *** |
| Error | 4.28 | 252 | 0.01698 | |
| Total | 57.78 | 314 |
*** P < 0.001
Two-way factorial ANOVAs of angular-transformed proportions of germinated seeds for the target species Lepidium sativum, Urtica dioica and Calamagrostis epigejos. The main effects of concentration (control, 2.5% and 5% dry leaf extract from Fallopia sachalinensis, Fallopia japonica and Fallopia ×bohemica) and light (white, green, dark) were not tested because the first-order interaction of the main effects (concentration) × (light) were statistically significant.
| Source of variation | Lepidium sativum | Urtica dioica | Calamagrostis epigejos | ||||||
|---|---|---|---|---|---|---|---|---|---|
| df | MS | F | df | MS | F | df | MS | F | |
| Concentration | 6 | 2.703 | 6 | 1.426 | 6 | 0.493 | |||
| Light | 2 | 0.907 | 2 | 0.682 | 2 | 0.260 | |||
| (Concentration) × (Light) | 12 | 0.142 | 7.13*** | 12 | 0.068 | 2.74** | 12 | 0.024 | 3.96*** |
| Error | 84 | 0.0199 | 84 | 0.0249 | 84 | 0.00613 | |||
*** P < 0.001** P < 0.01
Interactions of angular-transformed proportions of germinated seeds in the target species Lepidium sativum, Urtica dioica and Calamagrostis epigejos. The interactions between the concentration of aqueousextracts (control, 2.5 and 5% dry leaf extract from Fallopia sachalinensis, Fallopia japonica and Fallopia ×bohemica) and light intensity (white, green, dark) are apparent as non-parallel lines of light intensity at each panel. See Table 2 for statistical significance of these interactions.
For each target species and light regime, one-way ANOVAs indicated that the germination differed highly significantly among the individual levels of extract concentration from the three Fallopia taxa (Table 4). Germination of control seeds also differed highly significantly from that of seeds exposed to extracts, with seed from control samples germinating to higher percentages than those exposed to extracts, except in Urtica dioica in the dark. Thus, Urtica dioica in the dark was the only target species unaffected by phytotoxic effect of extracts.
One-way ANOVAs of mean angular-transformed proportions of germinated seeds at different concentrations of aqueousextract (control, 2.5% and 5% dry leaf extract from Fallopia sachalinensis, Fallopia japonica and Fallopia ×bohemica) and their orthogonal contrasts (control vs. extracts, Fallopia sachalinensis vs. Fallopia japonica vs. Fallopia ×bohemica, and 2.5% vs. 5% dry leaf extract from Fallopia sachalinensis, Fallopia japonica, and Fallopia ×bohemica, respectively) for the target species (Lepidium sativum, Urtica dioica and Calamagrostis epigejos) at different light intensity (white light, green light, dark). Degrees of freedom for ANOVAs among concentrations are df = 6, 28; for the contrast control vs. extracts df = 1, 28; for the contrast Fallopia sachalinensis vs. Fallopia japonica vs. Fallopia ×bohemica df = 2, 28; and for the 2.5% vs. 5% dry leaf extracts df = 1, 28.
| Source of variation | Lepidium sativum | Urtica dioica | Calamagrostis epigejos | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| White light | Green light | Dark | White light | Green light | Dark | White light | Green light | Dark | ||||||||||
| SS | MS | SS | MS | SS | MS | SS | MS | SS | MS | SS | MS | SS | MS | SS | MS | SS | MS | |
| Among concentrations | 5.489 | 0.915*** | 3.576 | 0.596*** | 8.863 | 1.477*** | 4.139 | 0.690*** | 2.375 | 0.396*** | 2.860 | 0.477*** | 1.044 | 0.174*** | 1.182 | 0.197*** | 1.025 | 0.171*** |
| Control vs. extracts | 0.919 | 0.919*** | 0.746 | 0.746*** | 2.495 | 2.495*** | 0.401 | 0.401** | 0.527 | 0.527*** | 0.0028 | 0.00280 ns | 0.0758 | 0.0758** | 0.297 | 0.297*** | 0.601 | 0.601*** |
| Fallopia sachalinensis vs. Fallopia japonica vs. Fallopia ×bohemica | 1.690 | 0.845*** | 1.681 | 0.840*** | 2.124 | 1.062*** | 2.427 | 1.213*** | 1.305 | 0.652*** | 1.473 | 0.736*** | 0.179 | 0.0896*** | 0.315 | 0.157*** | 0.145 | 0.0723* |
| 2.5% vs. 5% Fallopia sachalinensis | 1.608 | 1.608*** | 0.826 | 0.826*** | 1.087 | 1.087*** | 0.135 | 0.135 ns | 0.194 | 0.194** | 0.262 | 0.262*** | 0.385 | 0.385*** | 0.267 | 0.267*** | 0.135 | 0.135** |
| 2.5% vs. 5% Fallopia japonica | 0.00483 | 0.00483 ns | 0.0125 | 0.0125 ns | 1.593 | 1.593*** | 0.0293 | 0.0293 ns | 0.16 | 0.16** | 0.438 | 0.438*** | 0.0350 | 0.0350* | 0.00000 | 0.000000 ns | 0.0240 | 0.0240 ns |
| 2.5% vs. 5% Fallopia ×bohemica | 1.267 | 1.267*** | 0.311 | 0.311*** | 1.564 | 1.564*** | 1.146 | 1.146*** | 0.188 | 0.188** | 0.685 | 0.685*** | 0.369 | 0.369*** | 0.303 | 0.303*** | 0.120 | 0.120** |
| Error | 0.0175 | 0.0205 | 0.0218 | 0.0426 | 0.0183 | 0.0137 | 0.00577 | 0.00531 | 0.00731 | |||||||||
*** P < 0.001, ** P < 0.01, * P < 0.05, ns = not significant
Within taxa, in all significant cases, 2.5% extracts had lower inhibitory effect than 5% extracts. Extracts from Fallopia ×bohemica always produced highly significant differences in germination, and the same was true for extracts from Fallopia sachalinensis, except for the effect on Urtica dioica in the white light. Fallopia japonica exhibited the weakest difference between concentrations of the extracts: the difference was significant only in Lepidium sativum in dark, in Urtica dioica in green light and in dark, and in Calamagrostis epigejos in white light (Table 4).
Extracts from the three taxa, Fallopia sachalinensis, Fallopia japonica and Fallopia ×bohemica, always significantly differed in their effects on germination (Table 4). Ranked from the strongest to the weakest effect, the extracts from particular Fallopia taxa reduced germination in the order Fallopia sachalinensis > Fallopia ×bohemica > Fallopia japonica in Lepidium sativum and Urtica dioica, and Fallopia ×bohemica > Fallopia sachalinensis > Fallopia japonica in Calamagrostis epigejos.
Discussion Potential for allelopathic effects in Fallopia taxaAlthough the remarkably low species diversity in stands invaded by Fallopia
is probably due to the competitive advantage resulting from its tall
stature, rapid growth, dense rhizome system, and successful
regeneration from rhizomes (
While the effect of light regimes on germination
yielded inconclusive results, the interaction of light regime with the
inhibitory effect of leaf extracts indicates that poor germination and
establishment of native species in stands invaded by Fallopia
taxa is unlikely to be explained by the effect of shading alone.
However, our results demonstrate a pronounced phytotoxic effect of Fallopia
taxa on germination of native dominant species under laboratory
conditions. This inhibition presumably occurred through leaf chemical
compounds, was affected by light regime, and its outcome under
particular combination of factors depended on the species tested. As
pointed out by
Particular Fallopia taxa differed in their effect on germination of the target species. The weakest phytotoxic suppressor was Fallopia japonica;
its effect on both native dominants was generally lower than that of
its two congeners, and higher phytotoxic concentration often did not
result in a more pronounced effect. The weak phytotoxic effect of Fallopia japonica corresponds to generally lower regeneration (
This study was based on using dry leaves, in order to simulate the situation in the field; leaves fall off Fallopia
plants in dry conditions in the autumn and decomposition proceeds in
winter. Although the use of freshly collected and subsequently ground
dry leaf material in allelopathical studies has been criticised (
Our results, nevertheless, demonstrate that there is a strong potential phytotoxic effect of invasive Fallopia species on dominant native species and that this effect differs among the three taxa of this genus. Therefore, allelopathy cannot be excluded as one of the mechanisms contributing to the impact on the diversity of native species in Fallopia-invaded stands. The results of this paper further indicate that the light regime can influence the outcome of phytotoxic actions and should therefore be taken into account in studies focussing on allelopathic effects of plant species.
We thank Inderjit and Ingolf Kühn for valuable comments on an earlier draft of the manuscript, Hana Skálová and Radka Sudová for discussions on the topic and technical support, and Llewellyn Foxcroft for editing the English. The work was supported by a grant no. AV6005805/1998 from the Grant Agency of the Academy of Sciences of the Czech, by institutional long-term research plans no. AV0Z60050516 from the Academy of Sciences of the Czech Republic, and no. 0021620828 from MŠMT CR, and by the Biodiversity Research Centre no. LC 06073. Petr Pyšek acknowledges support by the Praemium Academiae award (AV0Z60050516) from the Academy of Sciences of the Czech Republic.