Research Article |
|
Corresponding author: John Hutchinson ( majmch@googlemail.com ) Academic editor: Ingolf Kühn
© 2014 John Hutchinson, Heike Reise, David Robinson.
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0) which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation:
Hutchinson J, Reise H, Robinson D (2014) A biography of an invasive terrestrial slug: the spread, distribution and habitat of Deroceras invadens. NeoBiota 23: 17-64. doi: 10.3897/neobiota.23.7745
|
 |
Abstract
The article reviews distribution records of Deroceras invadens (previously called D. panormitanum and D. caruanae), adding significant unpublished records from the authors’ own collecting, museum samples, and interceptions on goods arriving in the U.S.A. By 1940 D. invadens had already arrived in Britain, Denmark, California, Australia and probably New Zealand; it has turned up in many further places since, including remote oceanic islands, but scarcely around the eastern Mediterranean (Egypt and Crete are the exceptions), nor in Asia. Throughout much of the Americas its presence seems to have been previously overlooked, probably often being mistaken for D. laeve. New national records include Mexico, Costa Rica, and Ecuador, with evidence from interceptions of its presence in Panama, Peru, and Kenya. The range appears limited by cold winters and dry summers; this would explain why its intrusion into eastern Europe and southern Spain has been rather slow and incomplete. At a finer geographic scale, the occurrence of the congener D. reticulatum provides a convenient comparison to control for sampling effort; D. invadens is often about half as frequently encountered and sometimes predominates. Deroceras invadens is most commonly found in synanthropic habitats, particularly gardens and under rubbish, but also in greenhouses, and sometimes arable land and pasture. It may spread into natural habitats, as in Britain, South Africa, Australia and Tenerife. Many identifications have been checked in the light of recent taxonomic revision, revealing that the sibling species D. panormitanum s.s. has spread much less extensively. A number of published or online records, especially in Australia, have turned out to be misidentifications of D. laeve.
Keywords
Biological invasion, pest slug, Pulmonata, Agriolimacidae, Deroceras panormitanum
Introduction
Some terrestrial slugs and snails have been inadvertently spread by man well beyond their natural range (
The current paper gathers data on the spread of the slug Deroceras invadens Reise et al., 2011, which has often been reported (under various different names) as turning up in new places over the last century, thus revealing something of the colonisation process. Many relevant publications are widely dispersed in local journals, so there seems merit in reviewing these accounts in the hope of identifying global patterns. A better knowledge of the colonisation process may help in restricting or slowing the further spread of this and other slug species. The commonest sort of relevant data is the first occurrence in a country or administrative division. Unfortunately this is rather an unreliable statistic, because when a species first arrives its rarity makes its discovery very much a chance event, and because most local malacologists may not recognise the species until its first occurrence in their region has been published. Accordingly, we have tried also to assess rates of spread following the first discovery; unfortunately such monitoring is uncommon.
Our second object is to establish how far D. invadens has spread; several records of our own and unpublished information from museum collections significantly expand the known range. Conversely, some records turn out to be erroneous. Besides the value to those battling pest slugs in affected countries, a fuller knowledge of the range of climates that the species can tolerate may allow us to predict other regions that are at risk of being colonised. Thirdly we are interested in what habitats D. invadens occupies, particularly whether it invades agricultural and natural habitats. A fourth issue is how common the species gets, which can be assessed at various scales, such as proportion of grid squares occupied, proportion of sampling sites at which it is found, or number of animals collected. Here we will often compare with comparable data on D. reticulatum, a usually commoner congener that is found in similar synanthropic habitats but has spread earlier and more widely. Occurrence of D. reticulatum thus provides a proxy for estimating sampling effort (i.e., confirming the activity of someone interested in recording slugs).
The diversity of climates within the introduced range of D. invadens prompts the question of whether several cryptic species might be hidden within the diaspora. Therefore another aim has been to check the species identity of introduced populations. To understand the issues, it is helpful to review the taxonomic background. Deroceras is the largest genus of terrestrial slugs, with over 100 species described (
Material and methods
Besides checking literature in our own collections, we carried out online searches for “caruanae”, “panormitanum” and “invadens” particularly in combination with the names of specific countries. We also searched for the most recent species lists or distribution maps of likely host countries. We checked online museum catalogues, and personally screened the natural history museums in London and Wrocław. A.J. de Winter kindly selected relevant material from the Naturalis Biodiversity Centre, Leiden, and we have also borrowed material from the Rähle collection in the Stuttgart State Museum of Natural History, the Field Museum Chicago, the Florida Museum of Natural History, the Museum Victoria, the Queensland Museum, the Australian Museum, and the University Museum of Zoology Cambridge.
We have incorporated into the account results from our own fieldwork. Specimens collected by HR and JMCH are in the Senckenberg Museum of Natural History at Görlitz (SMNG). DGR has collected separately and specimens are in the collection of the U.S. Department of Agriculture (USDA) at the Academy of Natural Sciences in Philadelphia. Furthermore we have accessed the USDA collection of material intercepted arriving at U.S. ports (
HR confirmed identities using characters of the genitalia (
Literature commonly refers to D. invadens as D. caruanae, D. panormitanum or D. pollonerae (older literature also uses the genus name Agriolimax). Moreover, because the separation of D. invadens from D. panormitanum s.s. is recent, much literature is ambiguous to which species it refers. We have found it least confusing here to refer to all such ambiguous records as D. invadens, which is by far the more widespread species, rather than to distinguish unconfirmed records as “D. panormitanum s.l.” Table
List of countries and oceanic islands dealt with in the text (in the same order). The second column summarises the date D. invadens was first found (outdoors, unless specified). The ‘≤’ symbol indicates when a publication does not give a date of first collection. Dates of interception describe when the species was found on goods derived from that country. The third and fourth columns give the number of sites (or interceptions) for which we are sure that D. invadens rather than D. panormitanum s.s. occurs or vice versa; usually this evidence is our dissections, other cases are from publicly available COI sequences (indicated if this is the only evidence), otherwise the source is cited. We use ‘–’ instead of ‘0’ if there is no evidence of either species.
| Location | First known occurrence of D. invadens | Sites confirmed | |
|---|---|---|---|
| D. invadens | D. panormitanum s.s. | ||
| Europe | |||
| Italy | native | mainland 48, Sardinia 2, Sicily 5, Lipari Is >12 | mainland 1, Sicily 21 |
| San Marino | 2013 | 2 | 0 |
| Malta | no record | 0 | Malta 5, Gozo 2 |
| Great Britain | 1930 | England 25, Wales 5, Scotland 7 | Wales 1 ( |
| Island of Ireland | 1958 | 4 | 0 |
| France | ≤1945 | 12 | 0 |
| Monaco | 2012 | 1 | 0 |
| Belgium | 1968 | 8 | 0 |
| Netherlands | 1969 | 9 | 0 |
| Luxembourg | 1997 | 0 | 0 |
| Germany | 1979 | 18 | 0 |
| Switzerland | 1982 | 1 | 0 |
| Austria | ≤1977 | 2 | 0 |
| Czech Republic | 1996 | 1 | 0 |
| Slovakia | (greenhouse 2003) | 1 ( |
0 |
| Poland | 2001 | 1 | 0 |
| Lithuania | erroneous record | – | – |
| Hungary | no reliable record | – | – |
| Romania | erroneous record | – | – |
| Bulgaria | no reliable record | – | – |
| Greece | 2011 | 1 (COI) | 0 |
| Denmark | 1937 | 0 | 0 |
| Sweden | ≤1980 (greenhouse 1957) | 4 | 0 |
| Norway | 1983–84 (greenhouse c. 1967) | 2 | 0 |
| Finland | (greenhouse ≤1961) | 0 | 0 |
| Portugal | 1977 | 0 | 0 |
| Spain | 1974 | 12 | 0 |
| Africa | |||
| Egypt | 2005 | 0 | 0 |
| South Africa | 1963 | 2 | 0 |
| Kenya | interception 2012 | 1 interception | 0 |
| Asia and Australasia | |||
| Sri Lanka | erroneous record | – | – |
| Australia | 1936 | NSW 8, Victoria 4, Tasmania 1, S. Aus. 1, W. Aus. 2 | 0 |
| New Zealand | 1974, or maybe ≤1891 | 8 | 0 |
| Americas | |||
| USA | 1940 | Washington State 3, Oregon 2, California 4, Colorado 7, Utah 5, Washington DC 1 | 0 |
| Canada | 1974 (greenhouse 1966) |
British Columbia 10, Newfoundland 1 | 0 |
| Mexico | 1974 | 1 | 0 |
| Costa Rica | 2006 | 1 | 0 |
| Panamá | (interception 2007) | 2 | 0 |
| Colombia | 1975 | 1, 1 interception | 0 |
| Ecuador | 2012 (interception 2004) | 1 | 0 |
| Peru | (interception 2012) | 1 interception | 0 |
| Chile | ≤2003 | 3 | 0 |
| Argentina | 2010 | 1 (COI) | 0 |
| Brazil | 1991 | 1 interception | 0 |
| Oceanic islands | |||
| Faroe Islands | 1970 | 0 | 0 |
| Madeira | 1980 | 17 | 2 |
| Azores | 1957 | São Miguel 4, 1 interception | 1 interception |
| Canary Islands | 1947 | 9 | 0 |
| Tristan da Cunha | 1982–83 | 1 | 0 |
| Raoul Island | 1973 | 2 | 0 |
| Chatham Islands | 1976 | 0 | 0 |
| Marion Island | 1972 | 1 (COI) | 0 |
| Juan Fernández Islands | 1962 | 1 | 0 |
| Lord Howe and Norfolk islands | erroneous records | – | – |
Results
This section considers each country in turn. Countries are grouped by continent; within continents the ordering is mostly so that geographically close countries are dealt with together; Table
Europe
Italy and San Marino
The native range of D. invadens is thought to be in Italy (
Findings that significantly extend the range of D. invadens or D. panormitanum s.s. We exclude records published or available online in museum catalogues and Genbank. Identifications have been confirmed anatomically by HR except for those of Herdam; his notebooks show a sketch of the genitalia, but it is unclear if this is based on these East German specimens.
| Locality | Habitat | Latitude, Longitude | Collection date | Collector | Collection, catalogue number |
|---|---|---|---|---|---|
| D. invadens | |||||
| Hotel Garden Beach, Strada Panoramica Villasimius, Castiadas, Sardinia, ITALY | Unrecorded | c. 39.195°N, 009.561°E | 20.vi.2013 | S. Schnurrenberger | SMNG p17942 |
| SAN MARINO | Under bushes in town square, automatic irrigation | 43.9371°N, 012.4461°E | 14.iii.2013 | JMCH | SMNG p17943 |
| SAN MARINO | Olive grove | 43.9486°N, 012.4454°E | 16.iii.2012 | JMCH | SMNG p17944 |
| Above Av. de la Porte Neuve, Monaco-Ville, MONACO | Under bushes in park, automatic irrigation | 43.7316°N, 007.4222°E | 16.viii.2012 | HR, JMCH | SMNG p17945 |
| Tierpark, Berlin, GERMANY | Unrecorded | 52.50°N, 013.53°E | 1982 | V. Herdam | not preserved |
| Forstbotanischer Garten, Eberswalde, Brandenburg, GERMANY | Unrecorded | 52.83°N, 013.79°E | 1985 | V. Herdam | not preserved |
| Palmengarten, Frankfurt am Main, Hesse, GERMANY | Outdoors in alpine garden | c. 50.12°N, 008.65°E | 21.x.1985 | W. Hohorst | Hohorst collection in Senckenberg Museum für Naturkunde Frankfurt |
| Lentzeallee 94, Berlin, GERMANY | Suburban garden | 52.469°N, 013.303°E | 29.vii.2001 | HR | SMNG p10289 |
| Senzig, Brandenburg, GERMANY | Suburban garden | 52.286°N, 013.6590°E | 10.x.2004 | HR | SMNG p14038 |
| Enknachleiten, Braunau am Inn, AUSTRIA | Unrecorded | 48.25°N, 013.03°E | 5.iii.1999 | F. Seidl | Museum of Natural History, Wrocław University |
| Innsbruck, AUSTRIA | Track beside allotments | 47.2680°N, 11.4178°E | 12.iii.2013 | JMCH | SMNG p17946 |
| Hrádek nad Nisou, CZECH REPUBLIC | Suburban backyards | 50.85593°N, 14.84409°E, 50.85089°N, 14.84398°E | 3.vi.2014 | JMCH | SMNG p18006, p18007 |
| Bàscara, Catalonia, SPAIN | Beside spring | 42.16107°N, 002.90988°E | 24.viii.2012 | JMCH, HR | SMNG p17947 |
| Tudela, Navarra, SPAIN | On mudbank in canalised stream | 42.06353°N, 001.60082°E | 29.viii.2012 | JMCH | SMNG p17948 |
| Armidale, NSW, AUSTRALIA | Native grass pasture and scrub | 30.4339°S, 151.6750°E | 19.ix.2012 | M.A. Nash | SMNG p17986 |
| Nashdale, NSW, AUSTRALIA | Vineyard | 33.2962°S, 149.0209°E | 18.ix.2012 | MA. Nash | SMNG p17987 |
| Wallendbeen, NSW, AUSTRALIA | Canola/wheat field | 34.4987°S, 148.0509°E | 06.x.2012 | M.A. Nash | SMNG p17988 |
| Yass, NSW, AUSTRALIA | Wheat stubble | 34.7690°S, 149.1600°E | 06.x.2012 | M.A. Nash | SMNG p17989 |
| Mangoplah, NSW, AUSTRALIA | Canola field | 35.4516°S, 147.2631°E | 12.vii.2012 | M.A. Nash | SMNG p17990 |
| Stony Creek, Victoria, AUSTRALIA | Vegetable garden | 38.59°S, 146.06°E | 18.viii.2013 | G.M. Barker | SMNG p17991 |
| Mortlake, Victoria, AUSTRALIA | Canola field | 38.0221°S, 142.7569°E | 16.vi.2004 | M.A. Nash | SMNG p17992 |
| Dudley Peninsular, Kangaroo Is., SA, AUSTRALIA | Cliff top | 35.7328°S, 138.0036°E | 19.v.1985 | J. & F. Aslin, D. Adams | Field Museum Chicago 215867 |
| Porongurup, WA, AUSTRALIA | Wooded area in national park | c. 34.676°S, 117.869°E | 18.vi.1979 | A. Solem, F. & J. Aslin | Field Museum Chicago 204582 |
| Bothwell, Tasmania, AUSTRALIA | Wheat field | 42.3849°S, 147.0307°E | 28. vii.2013 | M.A. Nash | SMNG p17993 |
| Port Townsend, WA, USA | Town centre | 48.1158°N, 122.7546°W | 16.x.2001 | HR, JMCH | SMNG p17949 |
| Heart O' the Hills, S. of Port Angeles, WA, USA | Forest in National Park, near camp site | 48.040°N, 123.428°W | 17.x.2001 | HR, JMCH | SMNG p17950 |
| Priest Point Park, Olympia, WA, USA | Flower beds in park | 47.0693°N, 122.8940°W | 18.x.2001 | HR, JMCH | SMNG p17951 |
| Sunset Lake, Carnahan, OR USA | Street margins, front gardens | 46.100°N, 123.930°W | 3.x.2001 | HR, JMCH | SMNG p17952 |
| Oswego Lake, Portland, OR, USA | Beside stream | 45.4103°N, 122.6637°W | 3.x.2001 | HR, JMCH | SMNG p17953 |
| Phellan Gardens, 4955 Austin Bluffs Parkway, Colorado Springs, CO, USA | Greenhouse in garden centre | 38.9033°N, 104.7397°W | 23.vi.2004 | JMCH | SMNG p17954 |
| Arapahoe Acres Nursery, 9010 S. Santa Fe Drive, Littleton, CO, USA | Large garden centre | 39.5539°N, 105.0350°W | 24.vi.2004 | JMCH | SMNG p17955 |
| Cheesman Park, Denver, CO, USA | Suburban park, backing onto gardens | 39.730°N, 104.964°W | 21.vi.2004 | JMCH | SMNG p17956 |
| Paulino Gardens, 6300 N. Broadway, Denver, CO, USA | Large garden centre | 39.8097°N, 104.9856°W | 24.vi.2004 | JMCH | SMNG p17957 |
| Country Fair Garden Center, 2190 S Colorado Boulevard, Denver, CO, USA | Small urban garden centre | 39.6769°N, 104.9400°W | 24.vi.2004 | JMCH | SMNG p17958 |
| Fossil Creek Nursery, 7029 S. College Av., Fort Collins, CO, USA | Large garden centre | 40.4894°N, 105.0787°W | 26.vi.2004 | JMCH | SMNG p17959 |
| Arthur Ditch, Colorado State University, Fort Collins, CO, USA | Drainage ditch | 40.574°N, 105.087°W | vii.2004 | JMCH | SMNG p17960 |
| Western Garden Center, 550 S 600 E Salt Lake City, UT, USA | Garden centre | 40.7578°N, 111.8740°W | 11.viii.2006 | JMCH | SMNG p17961 |
| J & L Garden Center, 620 N 500 W, Bountiful, UT, USA | Garden centre | 40.8962°N, 111.8905°W | 18.viii.2006 | JMCH | SMNG p17962 |
| Park City, UT, USA | Gardens, urban street margins; 2150 m a.s.l. | 40.6438°N, 111.4952°W | 18.viii.2006 | JMCH | SMNG p17963 |
| Snowbird Resort, Little Cottonwood Canyon, UT, USA | Beside stream; 2460 m a.s.l. | 40.5805°N, 111.6560°W | 13–16.viii.2006 | JMCH | SMNG p17964 |
| Crescent Beach, White Rock, BC, CANADA | Gardens abutting beach | c. 49.054°N, 122.886°W | 14.x.2001 | HR, JMCH | SMNG p17965 |
| End of 92A Av., Langley, BC, CANADA | Cedar forest beside creek | 49.1706°N, 122.6523°W | 3.viii.2013 | JMCH | SMNG p17966 |
| Flume Rd, Robert’s Creek, BC, CANADA | Roadside ditch | 49.43085°N, 123.6654°W | 6.viii.2013 | JMCH | SMNG p17967 |
| Lyons Garden Centre, Salish Road at Halston Av., Kamloops, BC, CANADA | Garden centre | 50.7100°N, 120.3340°W | 23.vii.2013 | JMCH, RG & TJ Forsyth, HR | SMNG p17968 |
| Desierto de los Leones NP, Distrito Federal, MEXICO | Oak forest with sparse pines; 3000 m a.s.l. | 19.31°N, 099.31°W | 27.vii.1974 | A.S.H. Breure | RMNH.MOL.329841 |
| Tierra Blanca, Provincia Cartago, COSTA RICA | Under rocks, small wood in agricultural landscape, 2060 m a.s.l. | 09.9103°N, 083.8826°W | 17.ix.2006 | DGR | USDA 131032 |
| Boliva, Provincia Carchi, ECUADOR | On alder, 2600 m a.s.l. | 00.507°N, 077.900°W | 18.iv.2012 | L. Manangón | USDA 110614 |
| Vivero Limache, Comuna Limache, Provincia de Quillota, CHILE | In leaf litter, outdoors in nursery, 110 m a.s.l. | 33.005°S, 071.235°W | 14.iii.2008 | DGR | USDA 110169 |
| Jardin Japonés, Cerro San Cristóbal, Santiago, CHILE | In leaf litter, in urban park, 670 m a.s.l. | 33.4134°S, 070.6143°W | 12.iii.2008 | DGR | USDA 110153 |
| Granja Educativo de Lonquén, Comuna Talagante, CHILE | In leaf litter, outdoors in nursery, 350 m a.s.l. | 33.710°S, 070.873°W | 12.iii.2008 | DGR | USDA 110158 |
| Osorio, near Teror, Gran Canaria Island, CANARY ISLANDS | Agricultural area | c. 28.07°N, 015.54°W | 1984 | M. Ibáñez | SMNG p17969 |
| D. panormitanum s.s. | |||||
| Bordighera, Liguria, ITALY | Fallen leaves in concrete culvert. | 43.7853°N, 007.6826°E | 16.viii.2012 | JMCH, HR | SMNG p17970 |
| Below Cabo Girão, Municipo Cãmara de Lobos, MADEIRA | Under boulders along road | 32.659°N, 017.008°W | 1.i.2006 | DGR | USDA 131030 |
| Cabo Girão, Municipo Cãmara de Lobos, MADEIRA | Under rocks along road | 32.6571°N, 017.0018°W | 23.xii.2013 | DGR | USDA 140106 |
Table
Malta
Great Britain
Deroceras invadens was first found about 1930, in Cornwall, but by 1932 also from South Wales, central southern and northeast England, and Scotland (
The Conchological Society of Great Britain and Ireland publishes lists annually of vice-counties in the British Isles from which species have been newly recorded and the identity confirmed by experts. If we restrict attention to England and Wales (Scotland and Ireland were more sporadically recorded), the number of vice-counties increased only slowly to 13 by 1964, then jumped to 54 within 10 years (presumably at least partly an artefact of increased sampling effort in preparation of a distribution atlas). Over the next 25 years,
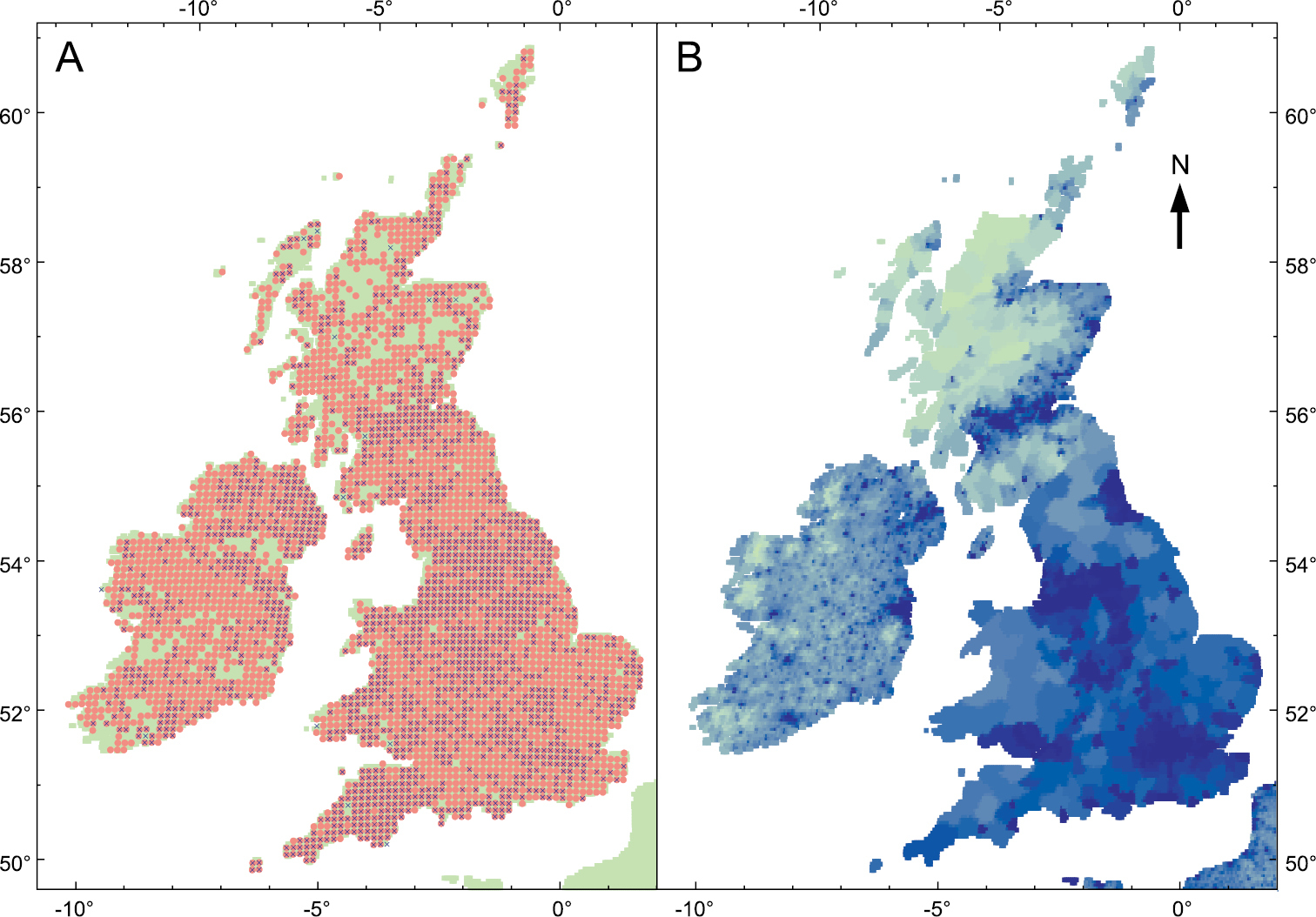
At a more local scale, D. invadens has been recorded from 1849 10 km grid squares in Great Britain; this is 0.44 as many as the almost ubiquitous D. reticulatum (access of NBN gateway on 24.xi.12; https://data.nbn.org.uk). The distribution map still shows some regions of scarcity (Fig.
The distribution of D. invadens in the British Isles. A Records of D. invadens (blue cross) and D. reticulatum (red circle) for each 10 km square (accessed from NBN gateway 24.xi.12) B Human population density (pale green = low, dark blue = high); sourced from Center for International Earth Science Information Network (Columbia University), Centro Internacional de Agricultura Tropical. 2005. Gridded Population of the World Version 3: Population Density Grids. Palisades, NY: Socioeconomic Data and Applications Center (SEDAC), Columbia University. Downloaded from http://sedac.ciesin.columbia.edu/gpw Nov. 2012.
Some regional differences in abundance are certainly not artefacts of recording intensity. For instance, in Suffolk (eastern England, sparsely populated) D. invadens was not reported until 1982 and a particularly thorough survey up to 1990 found it to be relatively scarce, with scattered localities across the county but concentrated in a couple of areas and generally only in gardens and disturbed ground (
Island of Ireland
The first records were in 1958, from several sites around Cork, and in 1959 from Newcastle, County Down, at the other end of the island and in a different country (
Today in Ireland the number of 10 km squares occupied by D. invadens is a similar proportion of those occupied by D. reticulatum as in Great Britain (0.38 vs 0.44; access to NBN gateway on 24.xi.2012; https://data.nbn.org.uk). In an interesting contrast with Britain,
France
In 1910 Simroth described Agriolimax scharffi collected in 1903 from La Giandola in the extreme southeast of France. Some (e.g.
The first clear reports of D. invadens from France are from
A map prepared in 1972 by
The first two records from Corsica were from 1977 (
Monaco
We believe that the first record of D. invadens from Monaco is our finding in 2012 under bushes in a park at a spot irrigated by an automatic watering system (Table
Belgium
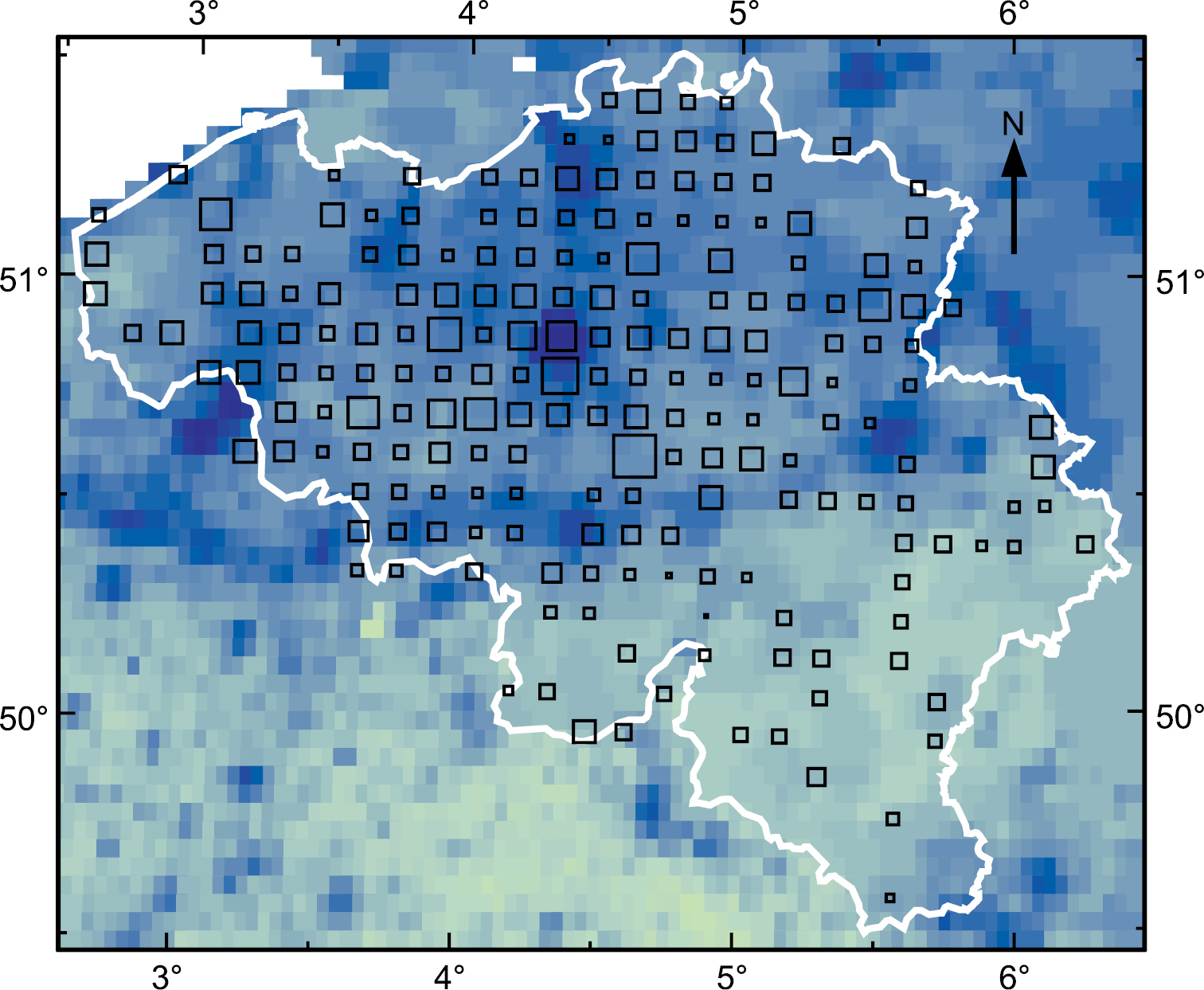
The first finding of D. invadens in Belgium was in 1968 in a Brussels garden (
Incidence of D. invadens in Belgium (white outline) 1968–83. Based on surveys by
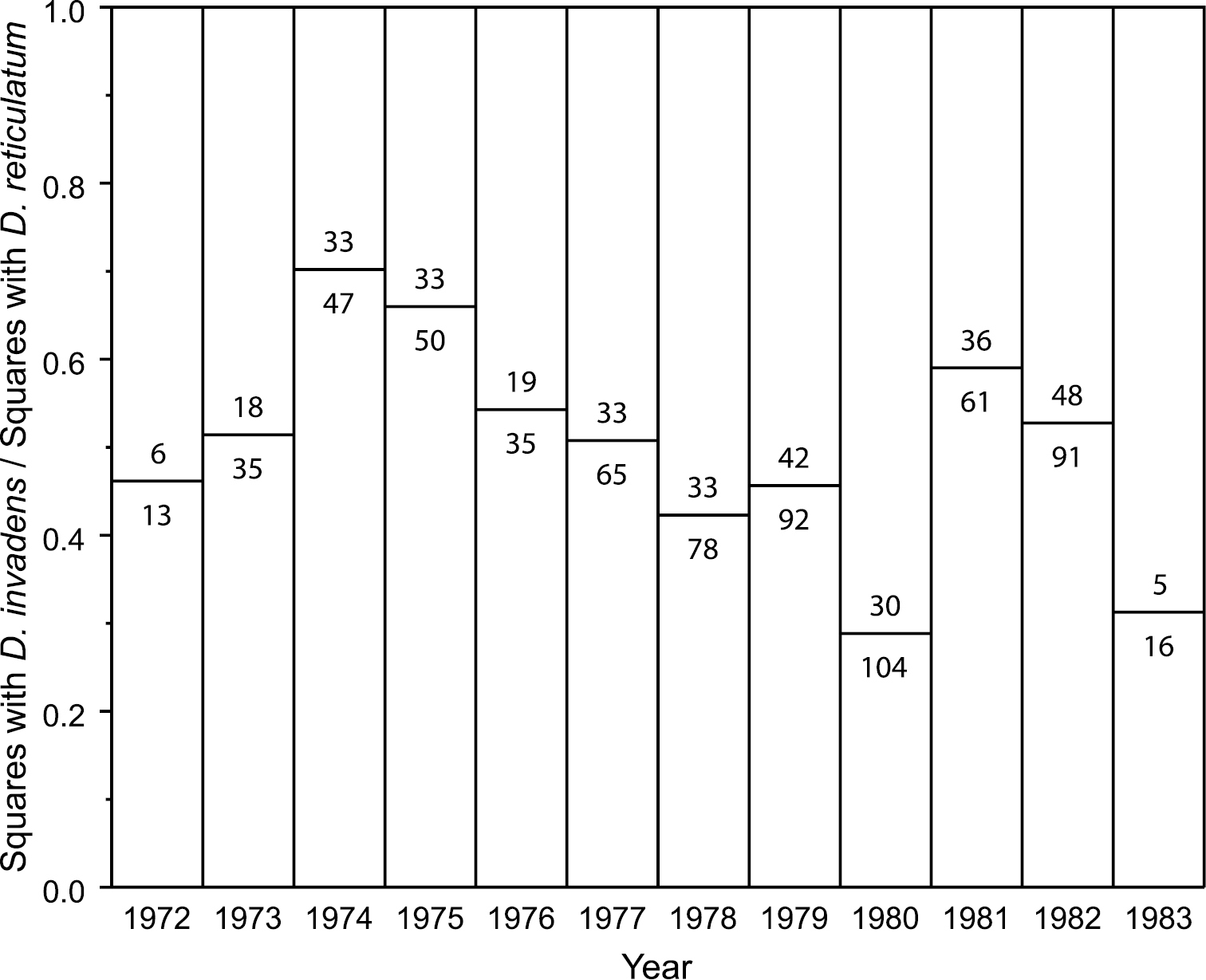
Additionally the Belgian data provide the number of grid squares in which each species of Deroceras was found each year. Surprisingly, the ratio between the counts for D. invadens and D. reticulatum does not increase over the period 1972–83 (Fig.
Deroceras invadens did not get easier to find in Belgium 1972–83. Ratio of the number of Belgian 10 km grid squares in which D. invadens was found to those in which D. reticulatum was found, for each year reported by
Netherlands
The first finding was in 1969 in Domburg at the southwest tip of the country (
Three intensive local surveys from the south of the Netherlands confirm the broad pattern. In Zuid-Holland,
Luxembourg
There are four 1997–98 records of D. invadens provided by Weitmann and Groh in the database of the Musée d’histoire naturelle Luxembourg (accessed via http://data.gbif.org/species/5190777 Sept. 2012).
Germany
Bavaria: first found in 1977 (see above). North Rhine Westphalia: first found in 1979, at several sites near Cologne (
Saxony: first found in 1990, in Limbach near Reichenbach (
A 2000–12 survey covering a broad area of northwest Germany, extending also into the Benelux countries, found that the mean density of D. invadens slightly exceeded that of D. reticulatum in gardens and in early successional woodland (
In conclusion, D. invadens is now widely distributed in Germany and can be a common synanthrope in the west, but it took decades to turn up in many states and is still uncommon in many areas. The other invading Deroceras, D. sturanyi, is often commoner (e.g.,
Switzerland
The first record was in Basle near the German border in 1982 (
Austria
The first record was in or before 1977 from a market garden in Maissau (NE Austria;
A 1992 survey of greenhouses around Vienna found D. invadens in 3 out of 10 establishments (the same as for D. reticulatum; D. laeve occurred in 6:
Czech Republic
A specimen collected in Ostrava in 1996 was identified as D. invadens only in 2003 (
Slovakia
Deroceras invadens is known only from greenhouses of the botanical garden in Bratislava where it was first found in 2003 (
Poland
The only record of which we are aware is from 2001 from the botanical garden and adjacent areas in Wrocław (SW Poland:
Lithuania
Deroceras invadens had been reported from the botanic garden in Kaunas (Skujienė G 2013 Invasive slugs in Lithuania: results, problems and perspectives of the investigations. Abstract booklet of “Slugs and snails as invasive species, a meeting of the IOBC/WPRS slugs and snails subgroup, Bergen, Norway 25–27 September 2013”, p. 11). However, Skujienė kindly lent us the specimen on which this record was based and it proved to have been misidentified.
Hungary
Deroceras invadens was listed as present in Hungary in the guide book by
Romania
Bulgaria
Greece
However, recently
Denmark
The first specimens were already collected in 1937, outdoors in a park in Odense. But their identity was not recognised until the mid 1950s when further specimens turned up at seven different cemeteries in another part of Denmark (northern end of Jutland:
See below for records from the Faroe Islands.
Sweden
The first findings date from 1957 to 1959, when D. invadens was found in six greenhouses well spread over the country (even up in Bysek at 65°N;
Norway
The first find was in about 1967 from a greenhouse in a botanic garden in Bergen (
Finland
Portugal
See below for records from Madeira and the Azores. The indications of D. panormitanum s.s., together with D. invadens, on both these archipelagos suggest that it would be worthwhile rechecking specimens from mainland Portugal.
Spain
The first published record of D. invadens was from Bilbao (north coast) in 1980 (
The first record for the Balearic Islands was 2001 from Majorca, where D. invadens has since been found in several other localities (
See below for records from the Canary Islands.
Africa
Egypt
A 2005–07 survey in Asyut Governate (along the Nile, upstream of Cairo) found D. invadens in 15 out of 38 gardens and farms (
South Africa
Deroceras invadens was recorded from several widely separated sites in Cape Province (George, Wilderness, Cape Town) in 1963–65 (
See below for records from Marion Island.
Kenya
A specimen was intercepted arriving in the USA on cut flowers (Astrantia) from Kenya (04.viii.12, USDA 110834). One area in Kenya where Astrantia is grown commercially for export is at Kipipiri, at an altitude of 2300–3000 m. Such altitudes may well provide a suitable habitat for D. invadens.
Asia and Australasia
Sri Lanka
Two publications report the presence of D. invadens in Sri Lanka (
Australia
Deroceras invadens has been reported widely from Australia (
We can confirm the occurrence of D. invadens in Victoria, New South Wales, Tasmania, South Australia and Western Australia (Table
Already in the 1975, D. invadens was described as “one of the commonest and most wide-spread of introduced slugs”, “a pest both of pasture plants and those of suburban gardens”, occurring “in only slightly disturbed native bushland as well as wholly modified habitats” (
See below for erroneous records from Lord Howe Island and Norfolk Island.
New Zealand
See below for records from Raoul Island and the Chatham Islands.
Americas
United States of America
With this firm foothold, one would expect the species to have become widely distributed in the USA, because some large horticultural firms grow plants in the benign climate of the Pacific Northwest and then ship nationally. However, compared with Europe, North America has far fewer experts able to identify slugs, especially those requiring dissection. Also problematic is that D. laeve in North America often grows larger than in Europe so that D. invadens is readily mistaken for it unless specimens are dissected (
In 1998 we found D. invadens in Washington DC, under bushes in a park, the first outdoor record in eastern North America (
The only other records from the eastern USA of which we are aware are from two sites in Kentucky (specimens in Florida Museum of Natural History, catalogue numbers 43778 and 44718, details available via http://data.gbif.org/species/5190777 accessed 24.iii.14). However, we have dissected one animal from each sample and they were not D. invadens.
Canada
The first Canadian records are from greenhouses in two cities in Quebec Province in 1966 (
Identifications of D. invadens from a garden in Edmonton, Alberta and from orchards by Osoyoos Lake, British Columbia, (
Mexico
We have confirmed the identification of specimens of D. invadens collected by A.S.H. Breure in 1974 at 3000 m in the Desierto de los Leones National Park, above Mexico City (Table
Costa Rica
In 2006, we found ten specimens under rocks in a small wood near Tierra Blanca, Provincia Cartago (USDA 131032; Table
Panamá
In July 2007, a specimen of D. invadens was found on a leaf imported into the USA from Panamá (USDA 131034). In July 2009, three further specimens were found on Allium imported into the USA from Panamá (USDA 131033). Note that Panamá connects Costa Rica to Colombia, countries for which the presence of D. invadens has been confirmed on the ground.
Colombia
Two specimens of D. invadens in the Field Museum Chicago (JK-198690, identity confirmed by HR) were collected by the University of Oxford expedition to Colombia in September 1975. There are no further locality data but in this month the expedition was both near Nazaret (Guajira state) and in the capital Bogotá (
Deroceras invadens was next found in 2000 at two rural sites near Bogotá in a garden and a flower plantation (
Cut flowers imported into the USA from Colombia in March 2008 contained D. invadens (USDA 131036).
Ecuador
In April 2012, L. Manangón collected a specimen “on alder” near Bolivar, Provincia Carchi (USDA 110614: Table
Peru
The only Deroceras species listed from Peru by
Chile
See below for a record from the Juan Fernández Islands. The presence of D. invadens there in 1962 suggests that it was probably present in mainland Chile by this time.
Argentina
Brazil
Recently (27.ii.14) a specimen of D. invadens was intercepted arriving in the USA on cabbage from a ship’s stores that had been loaded in Brazil (USDA 140148).
Oceanic Islands
Faroe Islands (Denmark)
This sizeable archipelago (1400 km2, population 50,000) lies between Scotland and Iceland at 62°N, having a maritime subarctic climate. In 1970,
Madeira (Portugal)
The first record of D. invadens is from 1980 (
Azores (Portugal)
A slug intercepted arriving in the USA from the Azores on a taro root in March 2008 was D. panormitanum s.s. (USDA 110434). So we checked specimens collected by J. Wieringa from four sites on São Miguel in 1987 (Naturalis Biodiversity Centre, Leiden: collection numbers 329842–329845). They were D. invadens, as was another U.S. interception from the Azores (USDA 131029, Dec. 2008). Probably, as on Madeira, both species occur.
Canary Islands (Spain)
The first records of D. invadens are from the island of La Palma in 1947 (
Tristan da Cunha (UK)
This lies in the middle of the South Atlantic (37.1°S), 2816 km from South Africa, with a population of under 300. The climate is temperate.
Raoul Island (New Zealand)
This lies 29.3°S in the South Pacific, 1100 km NNE of New Zealand’s North Island. The climate is subtropical. Deroceras invadens was found in 1973 in forest litter (
Chatham Islands (New Zealand)
These lie 44°S in the South Pacific, 680 km from New Zealand. The climate is temperate and they have a sizeable agricultural community and frequent transport links. Deroceras invadens was found in 1976 in pasture on the main island and nearby Pitt Island (
Marion Island (South Africa)
This is a subantarctic island (46.9°S, 290 km2) with a cool oceanic climate, unpopulated except for research stations; South Africa lies 1730 km to the northwest. Deroceras invadens was first reported in 1972, under timber and in damp mossy habitat beside the base hut; a thorough survey in 1965–66 had not reported it (
Juan Fernández Islands (Chile)
In the Field Museum Chicago and the Museum of Natural History, Wrocław University are specimens of D. invadens collected in 1962 from Robinsón Crusoe Island, formerly Más a Tierra (identities confirmed by HR; Field Museum catalogue number = 198633). This Pacific island is 48 km2 in area, and lies 600 km west of mainland Chile, with a mediterranean climate and a population in 1999 of over 500. The slugs were collected in a ravine in the Valle de Lord Anson, which rises from the main village.
Errors and Absences
Lord Howe Island and Norfolk Island are small (15 and 35 km2) but well populated Pacific islands, belonging to Australia although 570 and 1400 km east from the Australian mainland. Online records from the Australian Museum (http://ozcan.ala.org.au accessed 08.iii.2013) indicated that D. invadens was collected on Lord Howe Island in 2000 (single record) and on Norfolk Island in 1999 and 2002 (6 records, most from the largest patch of woodland but two from the opposite side of the island). We have borrowed the specimen from Lord Howe Island and two from Norfolk Island (one from each year); one from each island were D. laeve and the third specimen was not identifiable. Given the high rate of similar misidentifications of other Australian material (including from this museum; see above), we consider that there is no reliable evidence of D. invadens occurring on either island.
Specimens in the Natural History Museum London labelled as D. caruanae from São Tomé in the Gulf of Guinea (collected 1993 by A. Gascoigne from Lagoa Amelia and Tras-os-Montes; BMNH 19991797, 19991798) turned out to be D. laeve (dissection by HR); these records appear not to have been published or put online.
It may also be helpful to list some oceanic islands where D. invadens has not been found even though the climate might be suitable and recent surveys have been extensive and informed enough to have probably revealed the species were it well established: Iceland (
Discussion
Habitat
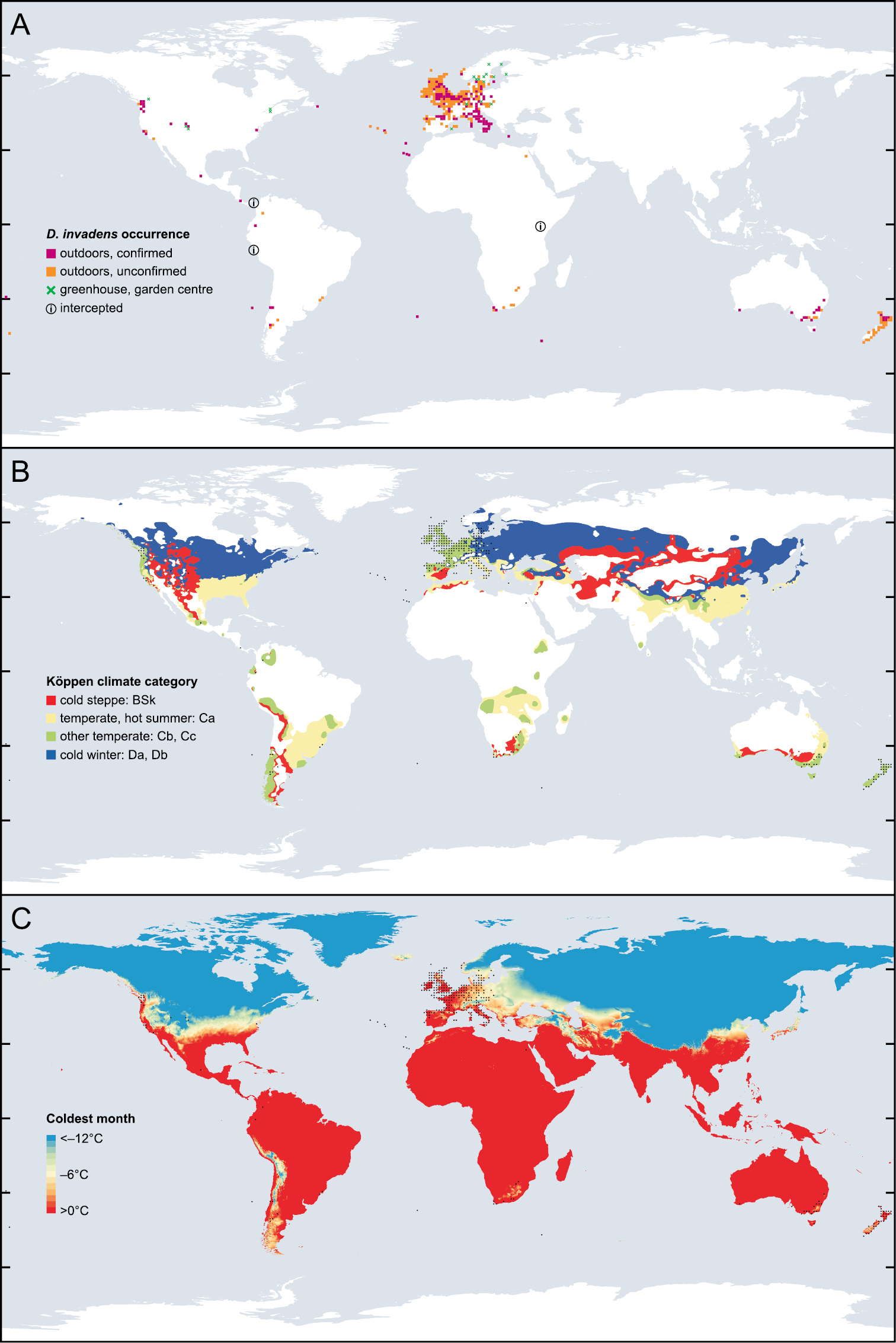
In Europe much of the area occupied by D. invadens counts as temperate according to the Köppen climate scale (Fig.
The global distribution of D. invadens related to climate. A Each symbol represents presence of D. invadens on a grid of one degree of latitude and longitude; exceptions are small oceanic islands (single symbol for each island group) and when records specify only a region which overlaps the grid lines (California, N Norway, Öland, and interceptions; a single symbol is marked in a representative “square”). Green cross = records only from greenhouse or garden centre; orange or magenta square = any other record (including garden or park); magenta indicates that at least one record has been confirmed to be D. invadens rather than D. panormitanum s.s.; circled i = only evidence of presence is interception on produce exported from that country. Swedish records in
Cold seems to be one critical factor. In the laboratory, D. invadens collected from Marion Island was unable to survive brief temperatures lower than –6.4 °C, or, on a longer time scale, temperatures lower than about –3 °C on average. This neatly explained its altitudinal range on that island (
In North America, three sites in Colorado and Utah where D. invadens has been found away from garden centres and plantings of annual bedding plants (Table
At the other extreme, too much heat is probably not a restriction per se, since the species occurs in Egypt, for instance.
In Central and South America, D. invadens occurs within the tropics but the accurately localised records are all from higher altitudes. This may be because higher altitudes tend to be cooler and have different precipitation patterns, or because such climatic differences have encouraged urbanisation or types of agriculture that favour the species. Higher altitudes would seem the likeliest place to search for D. invadens in other areas of the tropics, including Africa and India.
Deroceras invadens is typically associated with disturbed habitats, especially gardens, and is often easiest to find under discarded rubbish. It is one of the few molluscs to occur in the most urban sites, by surviving in the soil of flowerpots (
Deroceras invadens can become one of the most frequently encountered slugs, typically about half as frequent as D. reticulatum, but at some sites even commoner (e.g. Manchester gardens, British commercial greenhouses, Frankfurt am Main, Egypt). In the laboratory we have observed that D. invadens can mature a month faster than D. reticulatum, which may give it an advantage when growing seasons are short owing to either climate or agricultural activities.
Geographical range
Deroceras invadens is widespread over most of the western half of Europe. However, there are still areas within this region where it is scarce. In some cases this is probably because of climate (cold in Scandinavia, summer drought in central Spain), in other areas it might merely be because of a lack of time to spread there (e.g. Suffolk, Alsace). The species has yet to spread far in eastern Europe and is still much more thinly spread in Germany and Austria than in Belgium or Great Britain, for instance. Currently the most eastern outdoor records in Europe are the Baltic coast of Sweden, Wrocław in Poland and Ostrava in the Czech Republic; further east, at least in Lithuania, Latvia and the Ukraine, there is sufficient current interest in slugs that D. invadens would probably have been recorded had it become well established (
The species has long been known from the Pacific Northwest of America, and also occurs sporadically elsewhere in the USA and Canada. The pronounced scarcity of records in the east compared with the west is untypical of other introduced European slugs. The reason could be that much of the east has an unsuitable climate for D. invadens; perhaps in areas southern enough to for the winters not to be too cold, the summers are too hot and dry (Fig.
Elsewhere in the southern hemisphere, the species has been present, maybe for a long time, in the former British colonies of South Africa, Australia and New Zealand. In this context, the indication from interceptions that it may be present in Kenya is not surprising. It has also colonised a number of remote oceanic islands; the maritime influence on their climates is probably favourable, and perhaps also their depauperate faunas have left a niche vacant.
The range of D. invadens is impressive (Fig.
The similar species D. panormitanum s.s. from Sicily and Malta has also been introduced elsewhere, but much more rarely than D. invadens: the only such records are from one site in northern Italy, one in Wales, two adjacent sites on Madeira and an interception from the Azores (Tables
Spread
Deroceras invadens has been directly observed arriving from abroad on salads, vegetables, flowers, roots, and tiles (
We hoped that our review of the literature would illuminate the rate and pattern of these dispersal processes, but mostly it is hard to be sure that the apparent rate of spread is actually not the spread of awareness that this novel species is worth distinguishing from others. That is particularly a problem with a slug species that requires dissection for reliable identification. In several cases (e.g. Britain, France, New Zealand) the species was probably widespread before anyone was aware of its presence; presumably at a more local level the distribution continued to grow denser, but usually there are no follow-up surveys once someone has claimed the first record. What is really required is an initial survey reporting absences of the species, then comparable repeat surveys of the same places in subsequent years; this has rarely, if ever, been done.
There is nevertheless good evidence of a spread within one or two decades through the Azores and Tenerife. The German data are also probably reliable and representative in suggesting a time scale of one to two decades to extend over a larger country, but it is far from the case that every suitable garden or even district has been colonised within that time. Puzzling gaps in the present distribution elsewhere (e.g. Suffolk in England, Alsace in France) suggest that “filling in” can take decades longer. It is difficult to make quantitative comparisons between species, especially because the delay in spotting a new arrival depends on the ease of recognising the species, but D. invadens probably has spread a little slower than three other terrestrial molluscs that have also invaded much of Europe within the last century, the slugs Boettgerilla pallens Simroth, 1912 and Arion vulgaris (Moquin-Tandon, 1855) and the snail Hygromia cinctella (Draparnaud, 1801) (
One would expect uniparental reproduction to facilitate colonisation if adventitious human-mediated transport sometimes introduces a single slug at a time.
Acknowledgements
We thank the following for generously providing specimens: Jon Ablett, Roy Anderson, Gary Barker, Robert Forsyth, Jochen Gerber, Miguel Ibáňez, Darryl Potter, Richard Preece, Ted von Proschwitz, Mandy Reid, Wolfgang Rähle, Chris Rowley, Ulrich Schneppat, Bettina and Michael Schlitt, Sabrina Schnurrenberger, Willem Sirgel, Grita Skujienė, John Slapcinsky, Adrian Sumner, Ton de Winter, and the staff of Agrocalidad in Ecuador. Michael Nash deserves special thanks for his collecting in Australia for us and we would also like to acknowledge the diligence of numerous agricultural inspectors at US ports. Further thanks to the following for providing literature or other information: Roy Anderson, Gary Barker, Kevin Bonham, Ulrich Bössneck, Bram Breure, Arthur Chater, Benjamin Gómez, Eva Hackenberg, Shalika Kumburegama, Jürgen Jungbluth, Henk Mienis, Fred Naggs, Adrian Norris, Tello Neckheim, Barna Páll-Gergely, Ben Rowson, Alejandra Rumi. Ben Rowson, Ton de Winter, Bernhard Hausdorf, and a fourth anonymous referee all provided thoughtful reviews of the submitted manuscript and we are also grateful to Gary Barker, Robert Forsyth, and Anne Ludwig for their comments on it. We thank Bettina Schlitt for cataloguing specimens. A collecting trip to Italy was supported by the Paul Ungerer Foundation.
References
- Abeloos M (1945) Sur les formes néoténiques et microphalliques d’Agriolimax (Hydrolimax) laevis Müller. Bulletin de la Société Zoologique de France 70: 135–139.
- Aescht E, Bisenberger A (2011) Artenliste der Weichtiere (Mollusca: Gastropoda und Bivalvia) des Bundeslandes Oberösterreich mit Anmerkungen zur Gefährdung. Beiträge zur Naturkunde Oberösterreichs 21: 405–466. http://www.landesmuseum.at/pdf_frei_remote/BNO_0021_0405-0466.pdf
- Agudo-Padrón AI (2009) Recent terrestrial and freshwater molluscs of Rio Grande do Sul state, RS, southern Brazil region: a comprehensive synthesis and check list. Visaya Net August 2009: 1–13. http://www.conchology.be/documents/visaya-net/en/download.php?file=RECENT_MOLLUSCS_OF_RIO_GRANDE
- Agudo-Padrón AI, Lenhard P (2010) Introduced and invasive molluscs in Brasil: a brief overview. Tentacle 18: 37–41.
- Alexander KNA, Dubbeldam A (2013) A survey of ancient woodland indicator molluscs in selected sites on the Isle of Man. Journal of Conchology 41: 407–417.
- Alonso MR, Diaz JA, Ibáñez M (1986) Los pulmonados desnudos de las Islas Canarias. II. Superfamilia Limacoidea Rafinesque 1815. Vieraea 16: 97–112.
- Altena CO van Regteren (1950) The Limacidae of the Canary Islands. Zoologische Verhandelingen 11: 1–34. http://www.repository.naturalis.nl/document/148856
- Altena CO van Regteren (1966) Notes on land slugs 11: Arionidae, Milacidae and Limacidae from South Africa (Mollusca, Gastropoda, Pulmonata). Zoologische Mededelingen 41: 269–298 + 2 pl. http://www.repository.naturalis.nl/document/150268
- Altena CO van Regteren, Smith BJ (1975) Notes on introduced slugs of the families Limacidae and Milacidae in Australia, with two new records. Journal of the Malacological Society of Australia 3: 63–80. doi: 10.1080/00852988.1975.10673881
- Altonago K, Gómez B, Martín R, Prieto CE, Puente AI, Rallo A (1994) Estudio faunístico y biogeográfico de los moluscos terrestres del norte de la Península Ibérica. Parlamento Vasco (Vitoria–Gasteiz): 1–503.
- Anderson R (1983) Mapping non-marine Mollusca in north-west Ireland, 1976–1979. Irish Naturalists’ Journal 21: 53–62. http://www.jstor.org/stable/25538703
- Anderson R (1997) Species inventory for Northern Ireland: land and freshwater Mollusca. Environment and Heritage Service of Northern Ireland, Belfast, 1–26. http://www.doeni.gov.uk/wonderfulni/print/mollusc.pdf
- Anderson R (2003) Ganula lanuginosa (Boissy) and other additions to the fauna of Ibiza. Journal of Conchology 38: 95.
- Anderson R (2004) Pseudosuccinea columella (Say) and other additions to the fauna of Menorca. Journal of Conchology 38: 323.
- Anonymous (2003) Hardy nursery stock: integrated control of slugs and snails (Final project report). Department for Environment, Food and Rural Affairs, London, 1–8. http://randd.defra.gov.uk/Document.aspx?Document=HH1944TFV_1353_FRP.doc
- Anonymous (2011) Gough and Inaccessible Islands, United Kingdom of Great Britain and Northern Island. World Heritage Information sheets, maintained by UNEP–WCMC, 1–10. http://www.unep-wcmc.org/medialibrary/2013/04/09/b32c3378/WH_Information_Sheets_April2013.zip [Accessed 09.iv.2014]
- Backhuys W (1975) Zoogeography and taxonomy of the land and fresh-water molluscs of the Azores. Backhuys & Meesters, Amsterdam, i–vii, 1–350 + 97 maps + 32 pl.
- Bambaradeniya CNB (2002) The status and implications of alien invasive species in Sri Lanka. Zoos’ Print Journal 17: 930–935. http://www.zoosprint.org/ZooPrintJournal/2002/November/930-935.pdf
- Barker GM (1979) The introduced slugs of New Zealand (Gastropoda: Pulmonata). New Zealand Journal of Zoology 6: 411–437. doi: 10.1080/03014223.1979.10428382
- Barker GM (1982) Notes on the introduced terrestrial Pulmonata (Gastropoda: Mollusca) of New Zealand. Journal of Molluscan Studies 48: 174–181. http://mollus.oxfordjournals.org/content/48/2/174.full.pdf+html
- Barker GM (1990) Pasture renovation: interactions of vegetation control with slug and insect infestations. Journal of Agricultural Science 115: 195–202. doi: 10.1017/S0021859600075122
- Barker GM (1992) Naturalised terrestrial molluscs in New Zealand: origins and establishment. Proceedings, 41st Annual Conference of the Entomological Society of New Zealand: 54–62.
- Barker GM (1999) Naturalised terrestrial Stylommatophora (Mollusca: Gastropoda). Fauna of New Zealand No. 38. Manaaki Whenua Press, Canterbury, New Zealand, 1–253. http://www.landcareresearch.co.nz/__data/assets/pdf_file/0006/26295/FNZ38Barker19991.pdf
- Barrientos Z (2003) Lista de especies de moluscos terrestres (Archaeogastropoda, Mesogastropoda, Archaeopulmonata, Stylommatophora, Soleolifera) informadas para Costa Rica. Revista de Biología Tropical 51 (Suppl. 3): 293–304. http://www.redalyc.org/pdf/449/44911879014.pdf
- Beckmann K-H (2007) Die Land- und Süsswassermollusken der Balearischen Inseln. ConchBooks, Hackenheim, 1–255.
- Beckmann, K-H, Kobialka H (2008) Hygromia cinctella (Draparnaud, 1801) auf dem Eroberungszug durch Deutschland (Gastropoda: Hygromiidae). Club Conchylia Informationen 39: 34–41.
- Bieler R, Slapcinsky J (2000) A case study for the development of an island fauna: recent terrestrial mollusks of Bermuda. Nemouria 44: 1–99.
- Bishop MJ (1980) The distribution of recent terrestrial molluscs in Piemonte and Valle d’Aosta. Atti della Società Italiana di Scienze Naturali e del Museo Civico di Storia Naturale di Milano 121: 201–210.
- Bodon M, Boato A, Giusti F (1982) On the genus Deroceras in western Liguria, with description of a new species (Gastropoda: Derocerasidae). Animalia 9: 53–71.
- Boesveld A (2005a) Inventarisatie van de landslakken van Zuid-Holland. Stichting European Invertebrate Survey, Nederland, Leiden, 1–85. http://www.repository.naturalis.nl/document/46412
- Boesveld A (2005b) Inventarisatie van de landslakken van Noord-Brabant. Stichting European Invertebrate Survey, Nederland, Leiden, 1–104. http://www.repository.naturalis.nl/document/46413
- Boesveld A (2005c) Inventarisatie van de landslakken van de Zeeuwse kust, met de nadruk op de nauwe. Stichting European Invertebrate Survey, Nederland, Leiden, 1–25. http://www.repository.naturalis.nl/document/46411
- Borredà V, Collado MÁ (1996) Pulmonados desnudos (Gastropoda, Pulmonata) de la provincia de Castellón (E España). Iberus 14(2): 9–24. http://biodiversitylibrary.org/page/35323860
- Borredà V, Collado MA, Robles F (1990) Pulmonados desnudos de la provincia de Valencia. Iberus 9(1–2): 293–317. http://biodiversitylibrary.org/page/32631642
- Boschi C (2011) Die Schneckenfauna der Schweiz: ein umfassendes Bild- und Bestimmungsbuch. Haupt Verlag, Bern, 1–624.
- Bössneck U (1994) Deroceras panormitanum (Lessona & Pollonera, 1882) und Tandonia budapestensis (Hazay, 1881)—zwei für Ostdeutschland neue Nacktschneckenarten (Gastropoda: Stylommatophora: Agriolimacidae et Milacidae). Malakologische Abhandlungen Staatliches Museum für Tierkunde Dresden 17: 87–90.
- Boulord A, Douillard E, Durand O, Gabory O, Leheurteux E (2007) Atlas provisoire de la répartition des mollusques des Mauges (France, Maine-et-Loire). MalaCo 4: 184–221. http://www.journal-malaco.fr/documents/boulord_etal_malaco4.pdf
- Boyko CB, Cordeiro JR (2001) The terrestrial Mollusca of Easter Island (Gastropoda, Pulmonata). Basteria 65: 17–25.
- Bragado D, Araujo R, Aparacio T (2010) Atlas y libro rojo de los moluscos de Castilla–La Mancha. Organismo Autónomo Espacios Naturales de Castilla–La Mancha, Guadalajara, 1–506.
- Brander T, Kantee J (1961) Lounais-Hämeen nilviäiset, Mollusca. Lounais-Hämeen Luonto 11: 70–72.
- Breure ASH (1974) Notes on land and freshwater Mollusca from Southern and Central Mexico. I. De Kreukel 10: 131–148.
- Brodie G, Barker GM (2011) Introduced land snails in the Fiji Islands: are there risks involved? In: Veitch CR, Clout MN, Towns DR (Eds) Island invasives: eradication and management. IUCN, Gland, 32–36. www.iucn.org/dbtw-wpd/edocs/SSC-OP-042.pdf
- Brook FJ (2010) Coastal landsnail fauna of Rarotonga, Cook Islands: systematics, diversity, biogeography, faunal history, and environmental influences. Tuhinga: Records of the Museum of New Zealand Te Papa Tongarewa 21: 161–252. http://www.tepapa.govt.nz/SiteCollectionDocuments/Tuhinga/Tuhinga21_161_Brook.pdf
- Burke TE (2013) Land snails and slugs of the Pacific Northwest. Oregon State University Press, Corvallis, 1–344.
- Castillejo J (1983) Los pulmonados desnudos de Galicia, III. Estudio del género Deroceras Rafinesque, 1820 (Agriolimacidae, Gastropoda, Pulmonata). Iberus 3: 1–13. http://biodiversitylibrary.org/page/32802655
- Castillejo J (1997) Babosas del Noroeste Ibérico. Universidade de Santiago de Compostela, Santiago de Compostela, 1–192.
- Castillejo J (1998) Guia de las babosas ibéricas. Real Academia Galega de Ciencias, Santiago de Compostela, 1–154.
- Castillejo J, Rodríguez T (1991) Babosas de la Península Ibérica y Baleares: inventario crítico, citas y mapas de distribución: (Gastropoda, Pulmonata, Terrestria nuda). Monografías da Universidade de Santiago de Compostela No. 162. Universidade de Santiago de Compostela, Servicio de Publicacións e Intercambio Científico, Santiago de Compostela, 1–211.
- Chevallier H (1973) Repartition en France de Deroceras caruanae (Pollonera, 1891). Haliotis 3: 205–207.
- Chichester LF, Getz LL (1969) The zoogeography and ecology of arionid and limacid slugs introduced into northeastern North America. Malacologia 7: 313–346. http://biodiversitylibrary.org/page/13122502
- Chown SL, McGeoch MA, Marshall DJ (2002) Diversity and conservation of invertebrates on the sub-Antarctic Prince Edward Islands. African Entomology 10: 67–82. http://reference.sabinet.co.za/sa_epublication_article/ento_v10_n1_a7
- Cordoba M, Iglesias J, Ribadulla P, Castillejo J (2011) Performance of permanent refuge traps for the assessment of slug populations in pastureland. Annals of Applied Biology 159: 130–140. doi: 10.1111/j.1744-7348.2011.00481.x
- Cowie RH (1997) Catalog and bibliography of the nonindigenous nonmarine snails and slugs of the Hawaiian islands. Bishop Museum Occasional Papers 50: 1–66. http://hbs.bishopmuseum.org/pdf/op50.pdf
- Cowie RH (2001) Invertebrate invasions on Pacific islands and the replacement of unique native faunas: a synthesis of the land and freshwater snails. Biological Invasions 3: 119–136. doi: 10.1023/A:1014529019000
- Cucherat X, Demuynck S (2006) Catalogue annoté des Gastéropodes terrestres (Mollusca, Gastropoda) de la région Nord–Pas-de-Calais. MalaCo 2: 40–91. http://www.journal-malaco.fr/documents/cucherat&demuynck_Malaco2_2006.pdf
- Cunha RMT, Rodrigues A, Brito CP, Winnipenix B, Martins AMF (1994) Moluscos terrestres da Ilha do Faial. Lista preliminar. Relatórios e Comunicações do Departamento de Biologia da Universidade dos Açores 22: 16–19. http://hdl.handle.net/10400.3/872
- Cunha R, Rodrigues P, Martins A Frias (2010) List of molluscs (Mollusca). In: Borges PAV, Costa A, Cunha R, Gabriel R, Gonçalves V, Martins A Frias, Melo I, Parente M, Raposeiro P, Rodrigues P, Santos R Serrão, Silva L, Vieira P, Vieira V (Eds) A list of the terrestrial and marine biota from the Azores. Princípia, Cascais, 165–177.
- Dirzo R (1980) Experimental studies on slug–plant interactions. I. The acceptability of thirty plant species to the slug Agriolimax caruanae. Journal of Ecology 68: 981–998. doi: 10.2307/2259470
- Dvořák L, Čejka T, Horsák M (2003) First record of Deroceras panormitanum (Gastropoda, Agriolimacidae) from Slovakia. Biologia, Bratislava 58: 917–918.
- Ellis AE (1950) Recorder’s report: non-marine Mollusca. Journal of Conchology 23: 122–124.
- [Ellis AE] (1951) Census of the distribution of British non-marine Mollusca. Journal of Conchology 23: 171–244.
- Fabian Y, Sandau N, Bruggisser OT, Kehrli P, Aebi A, Rohr RP, Naisbit RE, Bersier L-F (2012) Diversity protects plant communities against generalist molluscan herbivores. Ecology and Evolution 2: 2460–2473. doi: 10.1002/ece3.359
- Falkner G (1979) Ein Freilandvorkommen von Deroceras (D.) panormitanum (Lessona & Pollonera) [= D. caruanae (Pollonera)] in Deutschland. Mitteilungen der Zoologischen Gesellschaft Braunau 3: 239–242.
- Falkner G (1982) Deroceras (D.) panormitanum (= D. caruanae) in der Schweiz. Mitteilungen der Zoologischen Gesellschaft Braunau 4: 134–135.
- Foltz DW, Ochman H, Selander RK (1984) Genetic diversity and breeding systems in terrestrial slugs of the families Limacidae and Arionidae. Malacologia 25: 593–605. http://biodiversitylibrary.org/page/13145716
- Forsyth RG (2004) Land snails of British Columbia. Royal British Columbia Museum, Victoria, Canada, i–iv, 1–188.
- Forsyth RG (2013) Towards an annotated catalogue of the terrestrial molluscs of Canada. The Malacologist 60: 22–23.
- Forsyth RG (2014) First record of Deroceras invadens Reise, Hutchinson, Schunack & Schlitt, 2011 (Gastropoda: Pulmonata: Agriolimacidae) from the island of Newfoundland, Canada. Check List 10: 149–150. http://www.checklist.org.br/getpdf?NGD141-13
- Forsyth RG, Hutchinson JMC, Reise H (2001) Aegopinella nitidula (Draparnaud, 1805) (Gastropoda: Zonitidae) in British Columbia—first confirmed North American record. American Malacological Bulletin 16: 65–69.
- Foster GN (1977) Problems in cucumber crops caused by slugs, cuckoo-spit insect, mushroom cecid, hairy fungus beetle and the house mouse. Plant Pathology 26: 100–101. doi: 10.1111/j.1365-3059.1977.tb01035.x
- Gavetti E, Birindelli S, Bodon M, Manganelli G (2008) Molluschi terrestri e d’acqua dolce della Valle di Susa. Monografie XLIV. Museo Regionale di Scienze Naturali, Turin, 1–273.
- Gittenberger E, Backhuys W, Ripken TEJ (1970) De Landslakken van Nederland. Koninklijke Nederlandse Natuurhistorische Vereniging, Amsterdam, 1–177.
- Giusti F (1976) Notulae malacologicae XXII. I molluschi terrestri, salmastri e di acqua dolce dell’Elba, Giannutri e scogli minori dell’Archipelago Toscano. Lavori della Società Italiana di Biogeografia 5: 99–355 + 19 pl.
- Glöer P, Hausdorf B (2001) Erstnachweise von Marstoniopsis scholtzi (A. Schmidt 1856) und Deroceras panormitanum (Lessona & Pollonera 1882) für Hamburg. Mitteilungen der Deutschen Malakozoologischen Gesellschaft 66: 9–12.
- Gómez BJ, Angulo E, Prieto CE (1981) Notas sobre algunos limacos (Arionidae, Limacidae, Milacidae) recogidos en los alrededores de Bilbao. Cuadernos de Investigación Biológica (Bilbao) 1: 21–25.
- Griffiths OL, Florens VFB (2006) A field guide to the non-marine molluscs of the Mascarene Islands (Mauritius, Rodrigues and Réunion) and the northern dependencies of Mauritius. Bioculture Press, Mauritius, i–xv, 1–185.
- Grimm FW, Forsyth RG, Schueler FW, Karstad A (2009) Identifying land snails and slugs in Canada: introduced species and native genera. Canadian Food Inspection Agency, Ottawa, i–iv, 1–168.
- Groh K (2012) Bibliography of the land and freshwater molluscs of the Cape Verde Islands, with a historical synopsis of malacological exploration in the archipelago and an annotated check-list. Zoologia Caboverdiana 3: 37–51. http://www.scvz.org/zoolcv/vol3no1/Groh%20Land%20snails.pdf
- Grossu AV (1969) Beschreibung einiger neuer Deroceras-Arten (Gastropoda, Limacidae). Archiv für Molluskenkunde 99: 157–170.
- Grossu AV, Lupu D (1965) Espèces nouvelles du genre Deroceras (Gastropoda, Limacidae) en Roumanie. Travaux du Muséum d'Histoire Naturelle “Grigore Antipa” 5: 25–31.
- Gural-Sverlova NV, Balashov IA, Gural RI (2009) Recent distribution of terrestrial molluscs of the family Agriolimacidae on the territory of Ukraine. Ruthenica 19: 53–61 [In Russian]. http://www.ruthenica.com/documents/VOL19_Sverlova_Balashov_Gural_53-61.pdf
- Gutiérrez Gregoric DE, Beltramino AA, Vogler RE, Cuezzo MG, Núñez V, Gomes SR, Virgillito M, Miquel SE (2013) First records of four exotic slugs in Argentina. American Malacological Bulletin 31: 245–256. doi: 10.4003/006.031.0204
- Hameury M-P (1958) Sur la présence en France de Deroceras caruanae (Pollonera 1891). Vie et Milieu 9: 81–87.
- Hanna GD (1966) Introduced mollusks of western North America. Occasional Papers of the California Academy of Sciences 48: 1–108 + 4 figs. http://biodiversitylibrary.org/page/3163753
- Hausdorf B (2002) Introduced land snails and slugs in Colombia. Journal of Molluscan Studies 68: 127–131. doi: 10.1093/mollus/68.2.127
- Hayes KA, Yeung NW, Kim JR, Cowie RH (2012) New records of alien Gastropoda in the Hawaiian Islands: 1996–2010. Bishop Museum Occasional Papers 112: 21–28. http://hbs.bishopmuseum.org/pubs-online/pdf/op112p21-28.pdf
- Hayward JF (1954) Agriolimax caruanae Pollonera as a Holocene fossil. Journal of Conchology 23: 403–404, pl. 15.
- Herbert DG (2010) The introduced terrestrial Mollusca of South Africa. South African National Biodiversity Institute (Pretoria): i–vi, 1–108. http://www.sanbi.org/node/1947
- Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A (2005) Very high resolution interpolated climate surfaces for global land areas. International Journal of Climatology 25: 1965–1978. doi: 10.1002/joc.1276
- Holland KD, McDonnell MJ, Williams NSG (2007) Abundance, species richness and feeding preferences of introduced molluscs in native grasslands of Victoria, Australia. Austral Ecology 32: 626–634. doi: 10.1111/j.1442-9993.2007.01749.x
- Holyoak DT (1983) Distribution of land and freshwater Mollusca in Corsica. Journal of Conchology 31: 235–251.
- Hommay G (2000) Quelques compléments sur les espèces de limaces présentes en Alsace. Bulletin de l’Association Philomathique d’Alsace et de Lorraine 36: 51–69.
- Horsák M, Dvořák L (2003) First records of the introduced slug Deroceras panormitanum (Lessona et Pollonera, 1882) from the Czech Republic (Mollusca: Gastropoda: Agriolimacidae). Folia Malacologica 11: 57–58. http://www.foliamalacologica.com/index.php?option=com_content&view=article&id=165&catid=56
- Horsák M, Juřičková L, Picka J (2013a) Měkkýši České a Slovenské republiky: molluscs of the Czech and Slovak Republics. Nakladatelství Kabourek Zlín, 1–264.
- Horsák M, Lososová Z, Čejka T, Juřičková L, Chytrý M (2013b) Diversity and biotic homongenization of urban land-snail faunas in relation to habitat types and macroclimate in 32 Central European cities. PLoS ONE 8: e71783. doi: 10.171/journal.pone.0071783
- Howlett SA (2005) The biology, behaviour and control of the field slug Deroceras reticulatum (Müller). Doctoral thesis, University of Newcastle upon Tyne. http://hdl.handle.net/10443/1640
- Iglesias J, Castillejo J, Castro R (2001) Mini-plot field experiments on slug control using biological and chemical control agents. Annals of Applied Biology 139: 285–292. doi: 10.1111/j.1744-7348.2001.tb00141.x
- Iglesias J, Castillejo J, Castro R (2003) The effects of repeated applications of the molluscicide metaldehyde and the biocontrol nematode Phasmarhabditis hermaphrodita on molluscs, earthworms, nematodes, acarids and collembolans: a two-year study in north-west Spain. Pest Management Science 59: 1217–1224. doi: 10.1002/ps.758
- Kappes H, Schilthuizen M (2014) Habitat effects on slug assemblages and introduced species. Journal of Molluscan Studies 80: 47–54. doi: 10.1093/mollus/eyt043
- Kappes H, Delgado JD, Alonso MR, Ibáñez M (2009) Native and introduced gastropods in laurel forests on Tenerife, Canary Islands. Acta Oecologica 35: 581–589. doi: 10.1016/j.actao.2009.05.004
- Kappes H, Katzschner L, Nowak C (2012) Urban summer heat load: meteorological data as a proxy for metropolitan biodiversity. Meteorologische Zeitschrift 21: 525–528. doi: 10.1127/0941-2948/2012/0361
- Kerney M (1999) Atlas of the land and freshwater molluscs of Britain and Ireland. Harley Books, Great Horkesley, Colchester, 1–261.
- Kerney MP, Cameron RAD, Jungbluth JH (1983) Die Landschnecken Nord- und Mitteleuropas: ein Bestimmungsbuch für Biologen und Naturfreunde. Parey, Hamburg, 1–384.
- Killeen IJ (1992) The land and freshwater molluscs of Suffolk: an atlas and history. Suffolk Naturalists’ Society, Ipswich, 1–171 + 18 pl.
- Klein Tank AMG, Wijngaard JB, Können GP, Böhm R, Demarée G, Gocheva A, Mileta M, Pashiardis S, Hejkrlik L, Kern-Hansen C, Heino R, Bessemoulin P, Müller-Westermeier G, Tzanakou M, Szalai S, Pálsdóttir T, Fitzgerald D, Rubin S, Capaldo M, Maugeri M, Leitass A, Bukantis A, Aberfeld R, van Engelen AFV, Forland E, Mietus M, Coelho F, Mares C, Razuvaev V, Nieplova E, Cegnar T, Antonio López J, Dahlström B, Moberg A, Kirchhofer W, Ceylan A, Pachaliuk O, Alexander LV, Petrovic P (2002) Daily dataset of 20th-century surface air temperature and precipitation series for the European Climate Assessment. International Journal of Climatology 22: 1441–1453. doi: 10.1002/joc.773
- Knappett CP, Mallet J, Sugden A (1976) Oxford expedition to the Serrania de Macuira, Colombia, 1975. Bulletin of the Oxford University Exploration Club, New Series 2: 7–19.
- Kofler A (1986) Zweiter Nachtrag zur Faunistik der Weichtiere Osttirols (Mollusca). Berichte des Naturwissenschaftlich-Medizinischen Vereins in Innsbruck 73: 71–86. http://www.landesmuseum.at/pdf_frei_remote/BERI_73_0071-0086.pdf
- Körnig G, Hartenauer K, Unruh M, Schnitter P, Stark A (2013) Die Weichtiere (Mollusca) des Landes Sachsen–Anhalt unter besonderer Berücksichtigung der Arten der Anhänge zur Flora-Fauna-Habitat-Richtlinie sowie der kennzeichnenden Arten der Flora-Fauna-Habitat-Lebensraumtypen. Berichte des Landesamtes für Umweltschutz Sachsen–Anhalt, Halle, 1–336.
- Kozłowski J, Kozłowski R (2011) Expansion of the invasive slug species Arion lusitanicus Mabille, 1868 (Gastropoda: Pulmonata: Stylommatophora) and dangers to garden crops—a literature review with some new data. Folia Malacologica 19: 249–258. doi: 10.2478/v10125-011-0005-8
- Kumburegama NPS, Ranawana KB (2001) Identification of pest snails and slugs of vegetable crops in four districts of Sri Lanka. Proceedings of the Annual Research Sessions, University of Peradeniya 6: 117.
- Lange WH (1944) Land slugs in California. Bulletin of the Southern California Academy of Sciences 43: 33–40. http://biodiversitylibrary.org/page/34148562
- Lee JE, Janion C, Marais E, Jansen van Vuuren B, Chown SL (2009) Physiological tolerances account for range limits and abundance structure in an invasive slug. Proceedings of the Royal Society B 276: 1459–1468. doi: 10.1098/rspb.2008.1240
- Leiss A, Reischütz PL (1996) Ein Beitrag zur Kenntnis der Molluskenfauna der Gewächshäuser in Wien und Niederösterreich. Wissenschaftliche Mitteilungen Niederösterreichisches Landesmuseum 9: 173–184. http://www.landesmuseum.at/pdf_frei_remote/WM_9_0173-0184.pdf
- Letelier S, Vega MA, Ramos AM, Carreño E (2003) Base de datos del Museo Nacional de Historia Natural: moluscos de Chile. Revista de Biología Tropical 51 (Suppl. 3): 33–137. http://www.redalyc.org/articulo.oa?id=44911879008
- Lill K (2001) Zur Verbreitung von Deroceras panormitanum, D. sturanyi, Candidula gigaxii und Monacha cartusiana in Niedersachsen und Bremen (Gastropoda: Stylommatophora: Agriolimacidae, Hygromiidae). Schriften zur Malakozoologie aus dem Haus der Natur, Cismar 17: 79–86.
- Lohmander H (1959) Faunistiskt fältarbete i västra och norra Jylland 1954–1957. Landmolluskerna. Göteborgs Musei Årstryck 1959: 33–104.
- McMillan NF (1972) Agriolimax caruanae Pollonera and other non-marine Mollusca in Faroe. Journal of Conchology 27: 419–421.
- Makings P (1959) Agriolimax caruanae Pollonera new to Ireland. Journal of Conchology 24: 354–356.
- Makings P (1962) Agriolimax caruanae in Ireland. Irish Naturalists’ Journal 14: 78–79. http://www.jstor.org/stable/25534865
- Martins AM de Frias, Cunha R Tristão da, Brito CP, Backeljau T (1990) Moluscos terrestres das Flores: lista preliminar. Relatórios e Comunicações do Departamento de Biologia da Universidade dos Açores 18: 39–45. http://hdl.handle.net/10400.3/843
- Martins AM Frias, Backeljau T, Cunha RM Tristão da, Brito CP (1991) Moluscos terrestres da Ilha de Santa Maria. Lista preliminar. Relatórios e Comunicações do Departamento de Biologia da Universidade dos Açores 19: 53–59. http://hdl.handle.net/10400.3/854
- Martins AM Frias, Cunha RM Tristão da, Rodrigues A, Brito CP (1993) Moluscos terrestres da ilha de São Jorge. Lista preliminar. Relatórios e Comunicações do Departamento de Biologia da Universidade dos Açores, 21: 55–60. http://hdl.handle.net/10400.3/907
- Meyer WM, Cowie RH (2010) Invasive temperate species are a threat to tropical island biodiversity. Biotropica 42: 732–738. doi: 10.1111/j.1744-7429.2010.00629.x
- Mienis HK (2003) Iets over de zuidelijke akkerslak Deroceras panormitanum en het voorkomen van deze geїntroduceerde naaktslak op Terschelling. Spirula 332: 61–62.
- Musson CT (1891) On the naturalised forms of land and fresh-water Mollusca in Australia. Proceedings of the Linnean Society of New South Wales (Series 2) 5: 883–896. http://biodiversitylibrary.org/page/3349181
- Naggs F, Raheem DC, Mordan PB, Grimm B, Ranawana KB, Kumburegma NPS (2003) Ancient relicts and contemporary exotics: faunal change and survivorship in Sri Lanka’s snail fauna. BCPC Symposium Proceedings 80: 103–108.
- Nash MA, Thomson LJ, Hofmann AA (2007) Slug control in Australian canola: monitoring, molluscicidal baits and economic thresholds. Pest Management Science 63: 851–859. doi: 10.1002/ps.1411
- Neckheim CM (2013) Collecting land and freshwater molluscs during a visit to Canada in 2010. Part one: Alberta. Spirula 392: 97–101.
- Neckheim CM (2014) Collecting land and freshwater molluscs during a visit to Canada in 2010. Part two: British Columbia. Spirula 396: 8–24.
- North MC, Bailey SER (1989) Distribution of Boettgerilla pallens in north-west England. In: Henderson IF (Ed) Slugs and snails in world agriculture. BCPC Monograph No. 41. British Crop Protection Council, Thornton Heath, 327–329.
- Obuid-Allah AH, Abdel-Tawab HS, El-Bakary Z, Abd El-Wakeli KF, El-Sanabany A (2008) A survey and population dynamics of terrestrial slugs (Mollusca, Gastropoda) at Assuit Governate, Egypt. Egyptian Journal of Zoology 51: 585–608.
- Olsen KM (2002) Landsnegler i Norge—en oppsummering og en presentasjon av tre nye arter, Oxychilus navarricus (Bourguignat, 1870), Lucilla singleyana (Pilsbry, 1890) og Hawaiia minuscula (Binney, 1840). Fauna 55: 66–77.
- Pearce TA, Mulvihill RS, Porter KA (2012) Land slugs (Gastropoda: Pulmonata) on birds demonstrate dispersal potential. Nautilus 126: 38–40.
- Pearce TA, Richart CH, Leonard WP, Hohenlohe PA (2013) Identification guide to land snails and slugs of western Washington. http://academic.evergreen.edu/projects/ants/TESCBiota/mollusc/key/webkey.htm [accessed 16.ix.13]
- Peel MC, Finlayson BL, McMahon TA (2007) Updated world map of the Köppen–Geiger climate classification. Hydrology and Earth System Sciences 11: 1633–1644. doi: 10.5194/hess-11-1633-2007
- Pilsbry HA (1948) Land Mollusca of North America (north of Mexico). Vol. II, Part 2. Academy of Natural Sciences of Philadelphia, Philadelphia, i–xlvii, 521–1113.
- Pintér L, Suara R (2004) Magyarországi puhatestűek katalogúsa: hazai malakológusok gyűjtései alapján. A magyarországi puhatestűek elterjedése II. Magyar Természettu-dományi Múzeum, Budapest, 1–547.
- Pollonera C (1891) Appunti di malacologia. VII.—Intorno ai Limacidi di Malta. Bollettino dei Musei di Zoologia ed Anatomia Comparata della Regia Università di Torino 6(99): 1–4. http://biodiversitylibrary.org/page/11673422
- Preece RC (1995) Systematic review of the land snails of the Pitcairn Islands. Biological Journal of the Linnean Society 56: 273–307. doi: 10.1111/j.1095-8312.1995.tb01091.x
- Preece RC (2001) Introduced land molluscs on the islands of the Tristan da Cunha–Gough group (South Atlantic). Journal of Conchology 37: 253–259.
- Proschwitz T von (1991) On the spread and development of the anthropochorous element in the land-snail fauna of the province of Dalsland (SW Sweden). Mitteilungen der Deutschen Malakozoologischen Gesellschaft 50/51: 15–31.
- Proschwitz T von (2002) Faunistikt nytt 2001—snäckor, sniglar och musslor. Göteborgs Naturhistoriska Museum Årstryck 2002: 29–46.
- Proschwitz T von (2009) Faunistical news from the Göteborg Natural History Museum 2008—snails, slugs and mussels—with some notes on the slug Limacus flavus (Linnaeus)—refound in Sweden, and Balea heydeni von Maltzan—a land snail species new to Sweden. Göteborgs Naturhistoriska Museum Årstryck 2009: 47–68.
- Proschwitz T von (2010) Faunistical news from the Göteborg Natural History Museum 2009—snails, slugs and mussels—with some notes on Pupilla pratensis (Clessin)—a land snail species new to Sweden. Göteborgs Naturhistoriska Museum Årstryck 2010: 41–62.
- QGIS Development Team (2013) QGIS Geographic Information System. Open Source Geospatial Foundation Project. http://qgis.osgeo.org
- Quick HE (1949) Synopses of the British Fauna. No. 8. Slugs (Mollusca). (Testacellidae, Arionidae, Limacidae). Linnean Society of London, London, 1–29.
- Quick HE (1960) British slugs (Pulmonata; Testacellidae, Arionidae, Limacidae). Bulletin of the British Museum of Natural History (Zoology Series) 6: 103–226 + 2 pl. http://biodiversitylibrary.org/page/2306260
- Rähle W (1992) Nacktschnecken (Arionidae, Milacidae, Agriolimacidae und Limacidae) von Madeira und Porto Santo (Mittelatlantische Inseln) (Gastropoda: Pulmonata). Malakologische Abhandlungen aus dem Staatlichen Museum für Tierkunde Dresden 16: 13–24.
- Ramírez R, Paredes C, Arenas J (2003) Moluscos del Perú. Revista de Biología Tropical 51 (Suppl. 3): 225–284. http://www.redalyc.org/articulo.oa?id=44911879012
- Reischütz PL (1977) Die Malakofauna des Waldviertels aus zoogeographischer Sicht. Jahres-Bericht des Bundesgymnasiums Horn 99: 4–9.
- Reischütz PL (1980) Beiträge zur Molluskenfauna des Waldviertels. In: Prihoda I (Ed) Festschrift zur 50-Jahr-Feier des Höbarthmuseum und Museumvereins in Horn 1930–1980. Museumverein, Horn, 259–275.
- Reischütz PL (1986) Die Verbreitung der Nacktschnecken Österreichs (Arionidae, Milacidae, Limacidae, Agriolimacidae, Boettgerillidae). Catalogus Faunae Austriae Suppl. 2. Sitzungsberichte der Östereichischen Akademie der Wissenschaften. Mathematisch-naturwissenschaftliche Klasse. Abteilung 1 195: 67–190.
- Reise H, Backeljau T (1994) Deroceras panormitanum (Lessona & Pollonera, 1882), sensu Giusti, 1986 in Ostsachsen (Gastropoda, Stylommatophora, Agriolimacidae). Abhandlungen und Berichte des Naturkundemuseums Görlitz 68: 71–75.
- Reise H, Hutchinson JMC, Forsyth RF, Forsyth T (2000) . The ecology and spread of the terrestrial slug Boettgerilla pallens in Europe with reference to its recent discovery in North America. Veliger 43: 313–318. http://biodiversitylibrary.org/page/42461023
- Reise H, Hutchinson JMC, Robinson DG (2006) Two introduced pest slugs: Tandonia budapestensis new to the Americas and Deroceras panormitanum new to the Eastern USA. Veliger 48: 110–115. http://biodiversitylibrary.org/page/42489826
- Reise H, Hutchinson JMC, Schunack S, Schlitt B (2011) Deroceras panormitanum and congeners from Malta and Sicily, with a redescription of the widespread pest slug as Deroceras invadens n. sp. Folia Malacologica 19: 201–223. doi: 10.2478/v10125-011-0028-1
- Ressl F (2005) Im Bezirk Scheibbs (NÖ) eingewanderte und eingeschleppte Tierarten an Beispielen einiger Nacktschnecken, Webspinnen, Asseln und Insekten. Wissenschaftliche Mitteilungen aus dem Niederösterreichischen Landesmuseum 17: 309–339. http://www.landesmuseum.at/pdf_frei_remote/WM_17_0309-0339.pdf
- Reuse C (1983) On the taxonomic significance of the internal shell in the identification of European slugs of the families Limacidae and Milacidae (Gastropoda, Pulmonata). Biologisch Jaarboek Dodonaea 51: 180–200.
- Reygrobellet D (1963) Une nouvelle espèce de limacidé, Deroceras meridionale n. sp. Bulletin de la Société zoologique de France 88: 399–402.
- Robinson DG (1999) Alien invasions: the effects of the global economy on non-marine gastropod introductions into the United States. Malacologia 41: 413–438. http://biodiversitylibrary.org/page/13111828
- Rodríguez T, Hermida J, Outeiro A (1993) La familia Agriolimacidae (Gastropoda, Pulmonata) en Portugal continental. Iberus 11(2): 35–44. http://biodiversitylibrary.org/page/32696481
- Rollo CD, Wellington WG (1975) Terrestrial slugs in the vicinity of Vancouver, British Columbia. Nautilus 89: 107–115. http://biodiversitylibrary.org/page/16319473
- Roth B, Sadeghian PS (2003) Checklist of the land snails and slugs of California. Santa Barbara Museum of Natural History Contributions in Science No. 3. Santa Barbara Museum of Natural History, Santa Barbara, 1–81.
- Ross HCG (1984) Catalogue of the land and freshwater Mollusca of the British Isles in the Ulster Museum. Ulster Museum, Belfast, i–vi, 1–160.
- Rowson B, Anderson R, Turner JA, Symondson WOC (2014a) The slugs of Britain and Ireland: undetected and undescribed species increase a well-studied, economically important fauna by more than 20%. PLoS ONE 9: e91907. doi: 10.1371/journal.pone.0091907
- Rowson B, Turner J, Anderson R, Symondson B (2014b) Slugs of Britain and Ireland: identification, understanding and control. Field Studies Council, Telford, i–vi, 1–136.
- Rudzīte M, Dreijers E, Ozoliņa-Moll L, Parele E, Pilāte D, Rudzītis M, Stalažs A (2010) Latvijas gliemji: sugu noteicējs. A guide to the molluscs of Latvia. LU Akadēmiskais apgāds, Rīga, 1–252.
- Schmid G (1997) “Malakologische Zuckungen”: Momentaufnahmen zur Molluskenfauna Baden–Württembergs. Veröffentlichungen für Naturschutz und Landschaftspflege in Baden–Württemberg 71/72: 719–858.
- Schnell P, Schnell W (1981) Deroceras caruanae (
Pollonera 1891 ) [Deroceras panormitanum (Lessona & Pollonera 1882)] und Deroceras sturanyi (Simroth 1894), zwei für das Rheinland neue Nacktschnecken (Gastropoda, Limacidae). Decheniana 134: 172–174.
- Seddon MB (2008) The landsnails of Madeira: an illustrated compendium of the landsnails and slugs of the Madeiran archipelago. Studies in Biodiversity and Systematics of Terrestrial Organisms from the National Museum of Wales. Biotir Reports 2. National Museum of Wales, Cardiff, i–viii, 1–196.
- Seixas MMP (1978) Descrição de uma espécie de Limacidae (Gasteropoda, Pulmonata), nova para a fauna portuguesa. Boletim da Sociedade Portuguesa de Ciências Naturais (2nd Series) 18: 5–6.
- Simone LRL (2006) Land and freshwater molluscs of Brazil. EGP, FAPESP, São Paulo, 1–390.
- Simroth H (1910) Nacktschneckenstudien in den Südalpen. Abhandlungen herausgegeben von der Senckenbergischen Naturforschenden Gesellschaft 32: 275–348, pl. 23–24. http://biodiversitylibrary.org/page/11717466
- Skujienė G (2002) Lehmannia valentiana (Férussac, 1823)—a newly introduced slug species in Lithuania (Gastropoda: Pulmonata: Limacidae). Acta Zoologici Lituanica 12: 341–344. doi: 10.1080/13921657.2002.10512521
- Smith BJ (1992a) Non-marine Mollusca. In: Houston WWK (Ed) Zoological Catalogue of Australia Volume 8. Australian Government Publishing Service, Canberra, i–xi, 1–399.
- Smith VR (1992b) Terrestrial slug recorded from sub-Antarctic Marion Island. Journal of Molluscan Studies 58: 80–81. doi: 10.1093/mollus/58.1.80
- Sneli J-A, Dolmen D, Solhøy T, Evertsen J, Høisæter T, Kjærstad G, Olsen KM, Schander C, Stokland Ø, Wikander PB, Økland J (2006) Bløtdyr: Mollusca. In: Kålås JA, Viken Å, Bakken T (Eds) Norsk Rødliste 2006—2006 Norwegian Red List. Artsdatabanken, Trondheim, 321–331. www.artsdatabanken.no/File/875/Norsk%20rødliste%202006
- Stanisic J, Shea M, Potter D, Griffiths O (2010) Australian land snails. Volume 1. A field guide to eastern Australian species. Bioculture Press, Mauritius, i–xii, 1–591.
- Sumner AT (2002) The Lothians garden survey. Conchologists’ Newsletter 161: 161–167.
- Sumner AT (2007) Slugs and snails in Iceland. Mollusc World 15: 6–7. http://www.conchsoc.org/MolluscWorld15/6
- Suter H (1913) Manual of the New Zealand Mollusca. John Mackay, Wellington, i–xxiii, 1–1120. doi: 10.5962/bhl.title.1716
- Tappert A (1996) Die Molluskenfauna von Köln. In: Hoffmann H-J, Wipking W, Cölln K (Eds) Beiträge zur Insekten-, Spinnen- und Molluskenfauna der Großstadt Köln (II). Decheniana Beihefte 35: 579–643.
- Thompson FG (2008) An annotated checklist and bibliography of the land and freshwater snails of Mexico and Central America: 1–903. http://www.flmnh.ufl.edu/malacology/mexico-central_america_snail_checklist [accessed 26.ix.13]
- Turner H, Kuiper JGJ, Thew N, Bernasconi R, Rüetschi J, Wüthrich M, Gosteli M (1998) Atlas der Mollusken der Schweiz und Liechtensteins. Fauna Helvetica 2. Centre Suisse de Cartographie de la Faune, Neuchâtel, 1–527.
- Unruh M (2001) Die Molluskenfauna des Burgenlandkreises. Schnecken und Muscheln—historische und gegenwärtige Übersicht. Saale–Unstrut-Jahrbuch 6: 86–99.
- Valovirta I (1967) List of Finnish land gastropods and their distribution. Annales Zoologici Fennici, 4: 29–32.
- Van Goethem JL (1974) Sur la presence en Belgique de Deroceras caruanae (Pollonera, 1891) et de Deroceras agreste (Linnaeus, 1758) (Mollusca, Pulmonata, Limacidae). Bulletin de l'Institut Royal des Sciences Naturelles de Belgique 50(2): 1–21.
- Van Goethem JL, De Wilde JJ, Marquet R (1984) Over de verspreidung in Belgie van de naaktslakken van het genus Deroceras Rafinesque, 1820 (Mollusca, Gastropoda, Agriolimacidae). Studiedocumenten Nr 14. Koninklijk Belgisch Instituut voor Natuurwetenschappen, Brussels, 1–45.
- Vernavá MN, Phillips-Aalten PM, Hughes LA, Rowcliffe H, Wiltshire CW, Glen DM (2004) Influences of preceding cover crops on slug damage and biological control using Phasmarhabditis hermaphrodita. Annals of Applied Biology 145: 279–284. doi: 10.1111/j.1744-7348.2004.tb00384.x
- Waldén HW (1960) Om ett par för Sverige nya anthropochora landmollusker, Limax valentianus Férussac och Deroceras caruanae (Pollonera) jämte några andra, kulturbunda arter. Göteborgs Kungliga Vetenskaps- och Vitterhets-Samhälles Handlingar, Sjätte Följden. Series B 6(8): 5–48.
- Waldén HW (2007) Svensk landmolluskatlas. Naturcentrum AB, Stenungsund, 1–271.
- Wardhaugh AA (1996) The terrestrial molluscan fauna of some woodlands in north east Yorkshire, England. Journal of Conchology 35: 313–327.
- Wiese V (1983) Zwei für Schleswig–Holstein neue Schneckenarten. Club Conchylia 15: 12–13.
- Wiese V (1991) Atlas der Land- und Süsswassermollusken in Schleswig–Holstein. Landesamt für Naturschutz und Landschaftspflege Schleswig–Holstein, Kiel, 1–251.
- Winter AJ de (1984) Over het voorkommen van Boettgerilla pallens Simroth en Deroceras panormitanum (Lessona & Pollonera) in Nederland. Correspondentieblad van de Nederlandse Malacologische Vereniging 219: 1547–1549.
- Winter AJ de (1988) Remarks on the non-marine molluscan fauna of the Azores. 1–2. Basteria 52: 105–109.
- Wiktor A (2000) Agriolimacidae (Gastropoda: Pulmonata)—a systematic monograph. Annales Zoologici 49: 347–590.
- Wiktor A (2001a) Deroceras (Deroceras) panormitanum (Lessona et Pollonera, 1882)—a new introduced slug species in Poland (Gastropoda: Pulmonata: Agriolimacidae). Folia Malacologica 9: 155–157. http://www.foliamalacologica.com/index.php?option=com_content&view=article&id=203&catid=78
- Wiktor A (2001b) Fauna Graeciae VIII. The slugs of Greece (Arionidae, Milacidae, Limacidae, Agriolimacidae—Gastropoda, Stylommatophora). Natural History Museum of Crete, Hellenic Zoological Society, Irakleio, i–viii, 1–241.
- Wiktor A (2004) Ślimaki lądowe Polski. Mantis, Olsztyn.
- Wiktor A, Szigethy AS (1983) The distribution of slugs in Hungary (Gastropoda: Pulmonata). Soosiana 10–11: 87–111.
- Zettler ML, Jueg U, Menzel-Harloff H, Göllnitz U, Petrick S, Weber E, Seeman R (2006) Die Land- und Süsswassermollusken Mecklenburg–Vorpommerns. Obotritendruck, Schwerin, 1–318.