(C) 2011 Joshua P. Atwood. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Research on post-establishment evolution in nonnative plant populations has focused almost exclusively on testing the Evolution of Increased Competitive Ability (EICA) hypothesis, which posits that the lack of specialized herbivores in the invaded range drives evolution in nonnative plant populations. Fifteen years of conflicting EICA test results suggest that selection pressures other than specialized herbivory are important in driving post-establishment evolution in invasive species. Alternative hypotheses, such as the Evolution of Reduced Competitive Ability (ERCA) hypothesis, have been proposed but have received little attention or testing. We argue that the lack of consensus across studies that test EICA may be due in part to the lack of consistent definitions and varying experimental design parameters, and that future research in this field would benefit from new methodological considerations. We examined previous work evaluating post-establishment evolution and evaluated the range of study systems and design parameters used in testing the EICA hypothesis. Our goal was to identify where different uses of ecological terms and different study parameters have hindered consensus and to suggest a path forward to move beyond EICA in post-establishment evolution studies. We incorporated these methods into a design framework that will increase data harmony across future studies and will facilitate examinations of any potential selection pressure driving evolution in the invaded range.
EICA, ERCA, invasion ecology, invasive plants, natural selection
It has been commonly observed that life-history traits of nonnative plant species vary across habitats in native and introduced ranges, most conspicuously as either increased growth (
Published hypotheses related to post-establishment evolution.
| Reference | Description |
|---|---|
|
|
Evolution of Increased Competitive Ability (EICA): Evolved increase in growth, decrease in defense associated with lack of herbivores in invaded range. |
| Sexton et al. 2002 | Invading populations benefit first from plasticity, then from local adaptation |
|
|
Latitudinal clines drive local adaptations in nonnative populations. |
|
|
Evolution of Reduced Competitive Ability (ERCA): Low amounts of plant competition result in an evolved decrease in growth, increase in reproduction, and defense. |
|
|
Multiple introductions and hybridization increase invasion success through increased genetic variability. |
| Blumenthal 2006 | Resource-Enemy Release Hypothesis (R-ERH): Resource availability effects how enemy release drives plastic and genetic trait variation. |
|
|
The absence of soil pathogens results in an evolved increase in growth. |
Because research regarding post-establishment evolution has been so tightly focused on testing the EICA hypothesis, much of the literature in this field evaluates a single reduced selection pressure: the lack of specialized herbivores in the invaded range. However, the inconclusive support found for the EICA hypothesis suggests that factors other than herbivore release may drive post-establishment evolution in some systems (
There have been a handful of studies evaluating selection pressures other than specialized herbivory, although they have not received the attention and scrutiny given to the EICA hypothesis.
Expanding our knowledge of post-establishment evolution beyond evaluations of the EICA hypothesis would address the omission of evolutionary potential from invasive species weed risk assessments, which attempt to predict the impact of an invading species on a given habitat based on the combination of species traits and habitat characteristics (
We examined previous work regarding post-establishment evolution in order to better understand why outcomes across studies have been inconsistent. Because this literature has focused primarily on testing the EICA hypothesis, we focused our analysis within the field of evolution and invasive species by specifically examining tests of the EICA. Unlike previous reviews, we specifically evaluated study systems and methodologies in order to identify design parameters that will allow better synthesis across research on post-establishment evolution. We also focused on the use of the term “competitive ability” and how its definition varied based on the context of experimental designs. We searched for relevant literature published since the introduction of EICA that explicitly tested the predictions of the EICA hypothesis in a common garden or reciprocal transplant design, resulting in 58 studies. We focused on common garden and reciprocal transplant designs because of their frequent use and their ability to minimize the effects of phenotypic plasticity in the examination of evolved trait differentiation. We reviewed each study and recorded information regarding study systems and design parameters, including whether abiotic and biotic variables were reported for seed collection sites, the inclusion of introduction history, sample size, the traits measured and metrics used, and the incorporation (or lack thereof) of competition in experimental manipulations. We also noted whether each study ultimately found support for the predictions of the EICA hypothesis (Table 2).
Design parameters of studies testing the EICA hypothesis. In the column labeled “Abiotic data, ” “1” indicates that researchers tried to incorporate a variety of abiotic environments in seed collection sites while “2” indicates that researchers tried to utilize similar environments. In the column “Traits, ” “G” = vegetative growth, “T” = herbivore tolerance, and “R” = reproductive effort. The column labeled “Comp.” indicates whether or not competition was incorporated into experimental manipulations. For “Metrics, ” growth metrics are denoted by “B” = biomass, “H” = height, “NL” = number of leaves, “ “LA” = leaf area, “BA” = basal area; tolerance metrics are denoted by “HM” = herbivore mass, “HA” = herbivore abundance, “LD” = leaf damage, “DC” = defense chemicals, “TD” = trichome density; reproductive metrics are denoted by “RM” = reproductive mass, “ReMR” = reproductive mass ratio, and “NF” = number of fruits. In the column “Sample Size, ” the values correspond to the number of native and nonnative populations used, respectively, unless the sample size was not differentiated by range. The column “EICA” indicates whether support was found, with “Partial” indicating that multiple traits were tested but not all results supported predictions, and “1” indicating that support was found, but only when plants were grown in the absence of competition.
| Article | Abiotic data | Intro. Hist | Traits | Comp. | Metrics | Sample size | EICA |
|---|---|---|---|---|---|---|---|
|
|
No | Yes | G T R | No | B, HA, RM | 8&22 | Yes |
|
|
Yes | No | G | No | B, ReMR | 3&3 | Yes |
|
|
No1 | No | G T R | Yes | NL, TD, RM | 20&20 | Yes |
|
|
No | Yes | G T | No | B, HA | 13&23 | Yes |
|
|
No | Yes | G T | No | B, H, HM | 1&1 | Yes |
|
|
No2 | No | G | Yes | B | 2&2 | Yes1 |
|
|
Yes2 | Yes | G R | Yes | H, B, RM | 8&8 | No |
|
|
Yes | Yes | G R | No | B, NF | 11&12 | No |
|
|
No | Yes | R | No | RM | 6&5 | Yes |
|
|
Yes2 | Yes | G T R | No | B, NL, NF | 1&1 | Partial |
|
|
Yes1 | Yes | G | No | LA | 4&4 | Yes |
|
|
No | Yes | G T | No | B, LA | 3&3 | No |
|
|
No | Yes | T | No | DC | 4&7 | No |
|
|
Yes1 | Yes | G | No | B, HA | 6&10 | No |
|
|
No | Yes | R | No | RM | 4&1 | Yes |
|
|
Yes | Yes | G | No | B | 4&4 | No |
|
|
No | Yes | T | No | DC | 4&3 | No |
| Erfmeier and Breulheide 2005 | Yes2 | Yes | G | No | H | 6&6 | Yes |
|
|
No | Yes | G T | No | B, HA | 1&1 | No |
|
|
No | Yes | G T | No | B, LD | 2&1 | No |
|
|
No | No | G | No | B, NL | 20&22 | Yes |
|
|
No | Yes | T | No | LD | 8&16 | No |
|
|
No | No | G | Yes | B | 8&9 | No |
|
|
No | Yes | G | No | B | 45 | No |
|
|
No | Yes | T | No | HM | 3&3 | No |
|
|
No | Yes | T | No | HM | 6&6 | Yes |
|
|
No | Yes | T | No | HM, DC | 10&20 | No |
|
|
No | Yes | T | No | DC | 10&22 | Yes |
|
|
No | Yes | T | No | DC, HM | 13&16 | Partial |
|
|
No | No | T | No | HA | 6&4 | No |
|
|
No | Yes | G T | No | B, LD | 1&1 | No |
|
|
No1 | Yes | T | No | HM | 7&4 | No |
|
|
Yes2 | Yes | G | Yes | B | 10&10 | Yes1 |
|
|
No | No | R | No | HA | 1&6 | No |
|
|
No | Yes | G R | No | B, NF | 18&32 | No |
|
|
Yes | Yes | G | Yes | B | 10&10 | No |
|
|
No | Yes | G R | No | H, LA, ReMR | 10&20 | No |
|
|
No1 | Yes | G T | No | H, HM | 10&20 | Partial |
|
|
Yes | Yes | G T | No | B, DC | 11&10 | No |
|
|
No | No | G R | Yes | B, NF | 22&23 | Partial |
|
|
No | No | G | Yes | B | 1&1 | Yes |
|
|
No | Yes | T | No | HM | 1&1 | Yes |
|
|
No | No | T | No | NL | 1&1 | No |
|
|
No1 | Yes | G T | No | BA, DC | 1&1 | Yes |
|
|
No | Yes | G T | No | BA, LD, DC | 1&1 | Yes |
|
|
No | Yes | G T | No | H, LD, HM | 1&1 | Yes |
|
|
Yes | No | G T | No | LA, DC | 4&4 | Partial |
|
|
Yes2 | Yes | G R | No | # Branches, Flowers | 17&7 | Yes |
|
|
No | Yes | T | No | H | 9&10 | No |
| Vilà and Gimeno 2005 | Yes | Yes | R | No | NF | 30&20 | Yes |
|
|
No1 | Yes | G | Yes | B | 10&20 | No |
|
|
No | Yes | G | No | H | 7&8 | Yes |
|
|
Yes | Yes | G | No | Plant volume | 10&10 | No |
|
|
No | No | G | No | B, H | 10&10 | Yes |
|
|
No1 | No | G | No | B | 3&3 | No |
|
|
No | No | G | No | B, H | 4&4 | Yes |
| Zou, Rogers, and Siemann 2008 | No | Yes | T | No | B, LD | 9&9 | Yes |
|
|
No | Yes | G T | Yes | B, LD | 2&2 | Yes |
Based on our results, we developed a framework that can be used to move beyond EICA to evaluate a broad range of habitat characteristics that can act as selection pressures driving post-establishment evolution. Our intent is to facilitate future research that expands the consideration of potential selection pressures and encourages integration of results across study species and organisms.
Methodological variability in evaluating selection pressures: lessons from tests of the EICA hypothesisThe variation in sample sizes of both native and nonnative populations used in common garden research is one of the most readily apparent differences among studies of post-establishment evolution (Table 2). The logistical difficulties of obtaining individuals from both the introduced and invaded ranges likely contribute to the small number of populations used to represent each range in most studies. Though a handful of studies used ten or more populations in each range (e.g.,
Most of the studies we examined measured only one trait despite the fact that hypotheses focused on post-establishment evolution generally discuss multiple traits related to one another through energetic tradeoffs. The EICA hypothesis makes two predictions that were explicitly tested by
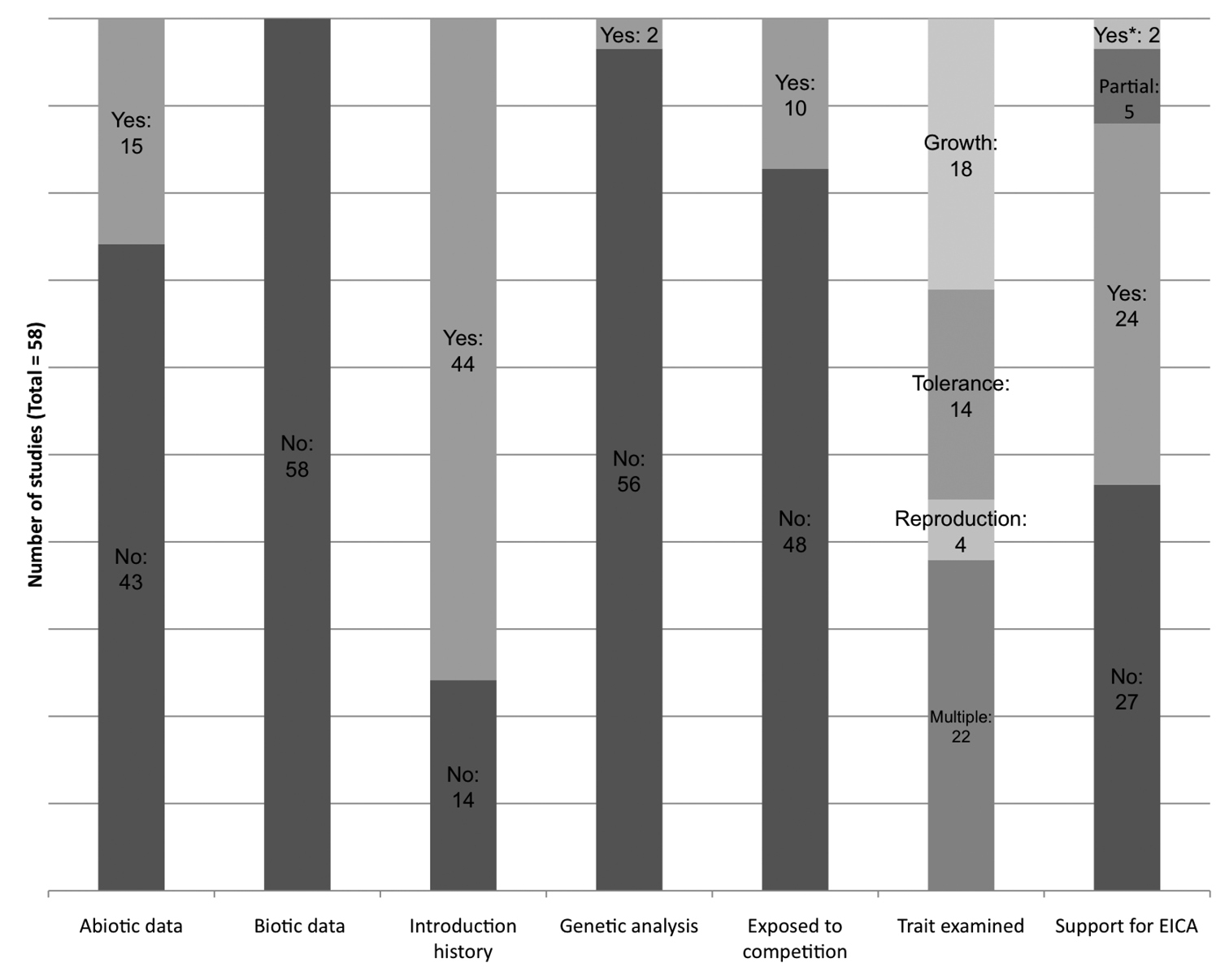
Frequencies of methodologies used in EICA studies. Data are aggregated from our review of design parameters detailed in Table 2. “*” Indicates that support for the EICA hypothesis was found only when plants were grown in the absence of competition.
Common garden designs also varied by study, either as outdoor gardens or greenhouse benches. Those studies that used outdoor gardens diverged further in whether plants were grown in pots (e.g.,
The term “competitive ability” had multiple interpretations across the studies that we reviewed. In studies where individuals are grown alone (e.g.,
These different definitions of competitive ability have also muddled our understanding of the energetic tradeoffs being examined in these studies. The focus on testing EICA has resulted in the general adoption of the term “competitive ability” as synonymous with “vegetative growth, ” as it was interpreted by
The use of different metrics for quantifying plant traits presents further challenges for data comparison across studies. While
The metrics used to quantify growth, herbivore tolerance and reproduction in studies examining post-establishment evolution. Data are aggregated from our review of design parameters detailed in Table 2.
| Trait | Metric |
|---|---|
| Growth | Total biomass |
| Aboveground biomass | |
| Belowground biomass | |
| Height | |
| Plant volume | |
| Basal area | |
| Leaf area | |
| Reproduction | Number of flowers |
| Fruit mass | |
| Herbivore Tolerance | Herbivore mass |
| Number of herbivores | |
| Defense chemical concentration | |
| Leaf damage |
In evaluating specialized herbivory as a selection pressure, studies of post-establishment evolution have generally neglected other habitat characteristics that can act as confounding variables across seed collection sites. Because comparisons of native and nonnative populations often use seeds collected on different continents, abiotic characteristics such as photoperiod or climate may vary significantly across the study area. For example, of the 58 studies we reviewed, only 15 identified differences in abiotic conditions (e.g., climate and/or photoperiod) between the native and invaded ranges. None of the studies we reviewed recorded information regarding the biotic characteristics of the collection site (e.g., plant community composition) other than differences in herbivore assemblages (Fig. 1, Table 2). This is particularly significant in light of a study by
Furthermore, the 15 studies that did report abiotic characteristics often disagreed as to whether consistency in abiotic factors across the sampling range was a desired component of the experimental design, despite having similar aims in evaluating evolution in nonnative populations. Several studies noted that seeds utilized in a common garden were intentionally collected from a wide variety of habitat types in order to incorporate environmental heterogeneity across the distribution of the species (e.g.,
Introduction history and subsequent spread of an invader are potentially influential factors often missing in reports of post-establishment evolution. This history is fundamental to understanding the selection pressures to which a species has been exposed. For example,
The broad range of approaches used in testing EICA may be one factor that limits consensus among the collective results. Twenty-four of the 58 studies we examined found support for the predictions of the EICA hypothesis, while 27 did not. Five studies found partial support, and two found support only when individuals were not exposed to competition (Fig. 1, Table 2). While EICA is likely an accurate predictor of evolutionary changes in some (but not all) of the species on which it has been tested, the fundamental differences in experimental designs and the use of loosely defined terms such as “competitive ability” in these studies may have hindered a more complete understanding of the applicability of the EICA hypothesis and of post-establishment evolution in general. While it is possible that meta-analyses could be used to make generalizations across methodologies (e.g.,
Post-establishment evolution is an area of research that has attracted substantial attention since the introduction of the EICA hypothesis, but the tests to date have not yet provided a meaningful consensus. One approach to facilitate progress in this field is to move towards standard definitions and comparable approaches that will more specifically evaluate potential selection pressures beyond the predictions of the EICA hypothesis.
Moving forward: A framework for designing evaluations of selection pressures in post-establishment evolutionBased on our analysis, we developed a framework for future research on selection pressures potentially driving evolution. We focused our recommendations on experimental designs that reduce the potential for confounding factors and increase the ability to integrate results among studies (Table 4).
Table 4. A framework for testing post-establishment evolution hypotheses.
| 1. | Evaluate the variability in abiotic and biotic conditions across ranges |
| 2. | Choose study species with appropriate life-history traits |
| 3. | Include introduction history |
| 4. | Incorporate competition in manipulations |
| 5. | Measure multiple traits and avoid the term “competitive ability” |
| 6. | Use standard metrics where possible |
Studies that examine a potential selection pressure should account for other habitat characteristics that may confound results. Ideally, the study system used for testing a post-establishment evolution hypothesis would use two ranges that are as similar as possible for all factors except for the characteristic being evaluated as a potential selection pressure. In particular, researchers can use seed collection sites at similar latitudes to partially control for photoperiod and climate (e.g.,
Study species selected to test post-establishment evolution hypotheses are ideally those that have a high potential for rapid evolution. Species that reproduce primarily by seed rather than clonal growth will have a higher frequency of genetic recombination, as will species with relatively short generation times such as herbaceous perennials. As such, r-selected species may be good candidates for studying post-establishment evolution, though we do not suggest that the role of K-selected species should be ignored. In testing hypotheses related to herbivore damage, it is appropriate to consider the relative effects of specialist and generalist herbivores on a given study species (e.g.,
Documenting the introduction and historic spread of a given species can reveal the types and durations of selection pressures that the species has undergone. The possibility of multiple introductions should also be considered since repeated introductions can increase genetic variation and/or result in novel genetic admixtures not found in the native range (
Because individuals in nature rarely grow in isolation, tests in which individuals are exposed to actual competition are likely to be more ecologically relevant. Incorporation of multiple competitive scenarios in common garden experiments will enhance our understanding of traits that are affected by competition. For example, growing individuals along a gradient of competitive stress (measured as the number of individuals per pot) would provide more information on the ability of a given species to obtain and utilize resources. The ratio of the number of individuals in a pot to soil volume can also be kept constant to avoid confounding competition with density (
Previous studies related to post-establishment evolution have used the term “competitive ability” as synonymous with vegetative growth, despite discussing growth in the context of energetic tradeoffs with other traits that may be affected by resource competition, including reproductive effort and herbivore tolerance (
The variety of metrics used to measure growth, reproduction, and herbivore defense has made it difficult to integrate data across studies. Utilizing standard metrics or converting units to those of standard metrics could facilitate such comparisons.
In studies measuring reproductive effort, a metric that demonstrates the energetic investment in reproductive biomass relative to total biomass is useful in accounting for the role of plant size in determining the amount of fruit produced. The reproductive mass ratio (ReMR=[fruit mass][total biomass]-1) has been used to describe the production of reproductive structures relative to total biomass production (
Compensatory growth response is a good candidate as a standard measure for quantifying the effect of herbivory. While many studies have used quantified defense chemical concentrations, not all plant species utilize the same chemical pathways. As a metric of response to herbivory, compensatory growth is relevant across all species that utilize different chemical pathways and therefore facilitates comparisons across studies.
Research on post-establishment evolution offers the potential for a better understanding of how nonnative plant populations interact with and adapt to their host environments. These data are relevant not only to invasion ecology, but also to studies of successional ecology and range expansions, as these fields also deal with new species interactions and novel habitat conditions (
Understanding how nonnative populations change over time is fundamental to their effective management, particularly with respect to weed risk assessments that attempt to predict the ways in which a given species might interact with a given habitat. Data on the evolutionary response of invading plant species can be used to incorporate evolutionary potential into such predictions, filling a knowledge gap that will allow researchers to predict not only the immediate impact of species invasions, but also how rapid evolutionary changes might over time alter the type or magnitude of those impacts (
We thank Elizabeth Farnsworth, Kimberly Lellis-Dibble, and two anonymous reviewers for comments and suggestions on early drafts of this manuscript.