(C) 2011 Arijana Barun. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
The small Indian mongoose (Herpestes auropunctatus) is one of the world’s 100 worst invasive species (
introduced predator, Apodemus, Crocidura
The small Indian mongoose (Herpestes auropunctatus) has been listed by the
Most mongoose introductions were in the late 19th and early 20th century to control introduced rats in sugar cane fields, but evidence of its success as a ratter is conflicting and mostly negative (
No comprehensive study has been devoted to the impact of the mongoose on the abundance of native small mammal populations, although several studies have proposed the mongoose as a major cause for the decline of species. For example,
On Adriatic Islands, the mongoose was introduced in 1910 to Mljet Island to control a poisonous viper (Vipera ammodytes) and subsequently to several other islands (Korčula in 1921 , Hvar (early 1950’s), Čiovo (ca. 1950’s), Škrda (ca. 1950’s), Kobrava (unknown) (
If introduced predators are capable of changing the abundance of their prey, conversely, prey may be able to assess predation risk and may behave accordingly, shifting their feeding, social, or escape behavior (
The goals of this study are: i) to assess the abundance of introduced rats and native small mammals on mongoose-infested and mongoose-free islands; ii) to compare rat activity times on mongoose-infested and mongoose-free islands, to test the hypotheses that activity times will be primarily diurnal where only the noctural marten is present (all the mongoose-free islands), but shifted towards night time when the diurnal mongoose is also present.
Methods Study area and field methodsWe conducted this study in 2008 on six islands in the southern part of Adriatic Sea: Lastovo (5, 300 ha), Brač (39, 400 ha), Dugi Otok (11, 400 ha), Mljet (10, 000 ha), Korčula (27, 000 ha) and Hvar (29, 900 ha). The first three are mongoose-free and the others are mongoose-infested. These islands are relatively similar in elevation, karst geology, Mediterranean climate and vegetation, but vary in surface area. They have a similar history of agricultural practices, human occupation, and timing of introduction of most exotic species. Their landscape is a fine-grained mosaic of small agricultural fields, scrublands (garrigue), thickets (maquis, mattoral), and forests. Agricultural production is mainly for local consumption and consists of olive groves and vineyards, with a few small vegetable fields with rich soil. A full description of these habitats is provided by
To determine small mammal abundance on every island, we set up three transects of 30 trapping spots distributed at 30 meter intervals in 900m long transects along narrow dirt roads, each running through all four vegetation types described previously in a proportion that may vary among transects. On each transect, trapping spots were placed alternatively on one side of the road and its opposite, and each trapping spot received two live traps: one INRA trap (stainless steel, horizontal bar-sprung trap similar to Sherman traps) to capture mammals weighing less than 30 g and one ratière trap (collapsible, wire and hanging bait-sprung trap,
To describe vegetation structure, four sample locations were evenly spaced along each transect, and the following data were collected within a 50-meter radius: % cover of bare ground, dead wood, rock, detritus, grasses in three layers (0–0.25 m, 0.25–0.5 m, 0.5–1 m); % cover of vegetation layers (0–0.25 m, 0.25–0.5 m, 0.5–1 m, 1–2 m, 2–4 m, 4–8 m, 8–16 m, 16–32 m, >32 m), maximum height of vegetation, canopy height, and % cover of each woody plant species. Within each vegetation layer, the relative cover was defined as the projection of the foliage volume of the layer on a horizontal plane. This was estimated by comparison with a reference percent cover chart (
We used PRIMER (Plymouth Marine Laboratory, UK) to conduct an analysis of similarity (ANOSIM) followed by pairwise comparisons to examine if two habitat variables (habitat characteristics and percent cover of each woody plant species) differed between islands with and without the mongoose. In the analysis, we nested six islands into two main grouping factors: mongoose present and mongoose absent. For each habitat variable, habitat characteristic, and percent cover of each woody plant species, we constructed a nonmetric multidimensional scaling (NMDS) plot, a nonparametric approach, using Bray–Curtis similarity coefficients from a triangular matrix (
To compare abundances of single species between islands with and without the mongoose, we calculated a Minimum Number Alive index (MNA) (
Sp1MNA = Sp1C/(NT-NTO – Sum AllSpp)
Sp1C is the number of captures for species one, NT is the total number of trap-nights, and NTO is the number of trap-nights the trap was inoperative for all species, whereas SumAllSpp is the total number of individuals of all other species captured.
To compare Rattus rattus and wood mouse (Apodemus sylvaticus) abundances between islands with and without mongooses, we calculated mean MNA indexes for each species for the three transects for each island and compared those values for the three islands with mongooses vs. the three mongoose-free islands with a t-test. To compare Rattus rattus activity times on mongoose-infested and mongoose-free islands, we performed Fisher’s exact test on the total number of captured rats for all three transects for each island, but we kept daytime captures separate from night captures. We performed all analyses in JMP, Version 8. (SAS Institute Inc., Cary, NC).
ResultsANOSIM indicated that composition of habitat characteristics did not differ between islands with the mongoose and islands without it (global R = 0.359, P = 0.136), nor did the percent cover of woody plant species differ (global R = -0.457, P = 0.115).
In Table 1 we list the mammal species found on each island according to
Mammalian species distributions on the islands under study, after
| Mongoose PRESENT | Mongoose ABSENT | |||||
| Mljet | Korčula | Hvar | Brač | Lastovo | Dugi Otok | |
| Herpestes auropunctatus | 31 | 21 | 5 | - | - | - |
| Martes foina | X | X | X | X | X | X |
| Canis aureus | - | X | - | - | - | - |
| Felis sylvestris (feral) | X | X | X | X | 1 | X |
| Rattus rattus | 158 | 83 | 62 | 55 | 44 | 97 |
| Mus musculus | 1 | X | X | X | X | X |
| Apodemus sylvaticus | - | 22 | 4 | 54 | 29 | 13 |
| Apodemus epimelas | 1 | X | - | - | - | - |
| Suncus etruscus | - | - | X | - | - | - |
| Crocidura suaveolens | 2 | 1 | 1 | 6 | 1 | 4 |
| Eliomys quercinus | - | 3 | X | X | X | - |
| Myoxusglis | X | X | X | X | - | - |
| Erinaceus concolor | X | X | X | X | X | - |
| Lepus europaeus | X | X | X | X | X | X |
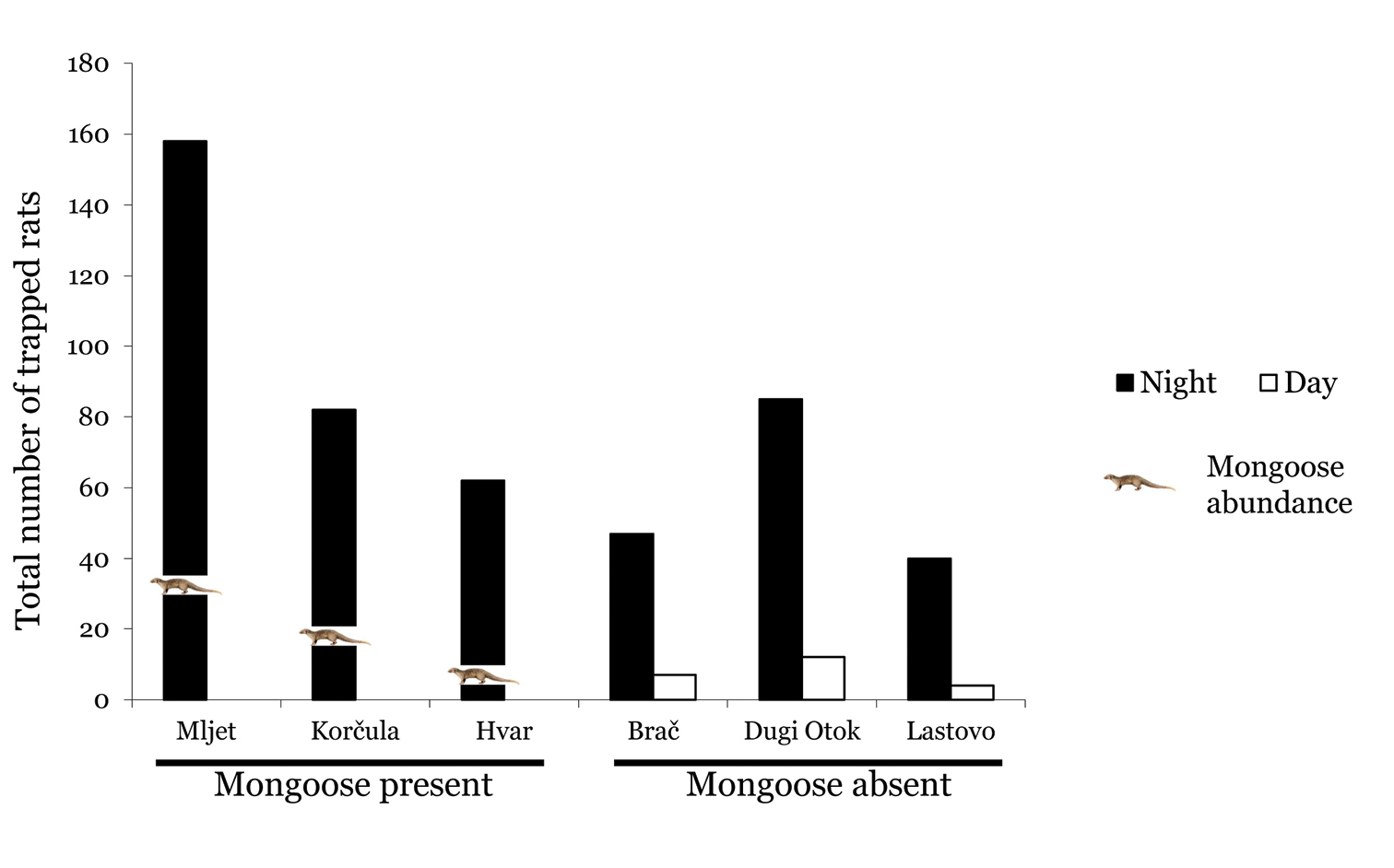
Mongooses were most abundant on Mljet and Korčula and much scarcer on Hvar (Fig. 1), where local hunters have conducted intensive, island-wide predator-control operations for several years (
The frequency of rats trapped during the day on mongoose-free islands exceeded that on mongoose-infested islands, (P < 0.001, Fisher’s exact test, Fig. 1); in fact no rats were trapped on mongoose-infested islands during the day.
Total number (April and May) of trapped rats during the night and day on three islands with the mongoose and three islands without the mongoose. Mongoose abundance is illustrated with the picture of a mongoose for each island.
Our data are too scant to allow a precise sense of the impact of the mongoose on small mammals on these islands. However, combined with previous work on the mongoose diet on these islands (
Although the INRA traps and the bait we used are effective for capturing Crocidura suaveolens (
As stated previously, the small Indian mongoose has frequently been cited as a species that could send already low island populations to the brink of extinction. In addition to the examples cited above, on Amami-Oshima Island, the shrew Crocidura orii is considered endangered because of the mongoose introduction (
As with Crocidura suaveolens, INRA traps and the bait used are efficient for capturing house mice on islands (
Experimental conditions and our protocol do not allow us to address rigorously the question of the specific consequences of the introduction of the two major alien species, Herpestes auropunctatus and Rattus rattus, on the native mammals. Nevertheless, the number of individuals captured of native species was more than three times greater on islands without the mongoose (107) than on islands with the mongoose (33); the number of Rattus rattus captures was one-third higher in the first situation (303) than in the second (196). This general trend suggests that at least one of the alien species has a detrimental effect on the native mammalian fauna, and probably both do.
In either case, our analyses show no statistical difference in Rattus rattus abundance on islands with and without the mongoose, and this result is in accordance with an already large but mostly speculative literature suggesting that, in spite of its reputation as a good ratter, the small Indian mongoose does not substantially control introduced Rattus rattus.
Our analyses show that the number of rats trapped during the day on mongoose-free islands exceeded those on mongoose-infested islands. This result accords with the proposed mechanism explaining the poor performance of the mongoose in reducing rat populations (
Procedures for research regarding capture and handling of animals followed the guidelines for the Institutional Animal Care and Use Committee at University of Tennessee (Approval Number 1373 v 11 7 07) and had permits from the Croatian Ministry of Culture (Approval Number 532-08-01-01/3-08-03). We thank Ivan Budinski and Antica Čulina for assistance in the field, Ivan Budinski for comments on the paper, James Fordyce, Nathan Sanders, Lara Souza and Frank VanManen for statistical advice, and the Department of Ecology and Evolutionary Biology, University of Tennessee for funding.