(C) 2012 Núria Gassó. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Success of invasive species has been frequently estimated as the present distribution range size in the introduced region. However, the present distribution range is only a picture of the invasion for a given time step and do not inform on the potential distribution range of the species. Based on niche-based models we used climatic, geographic and landscape information on the present distribution range for 78 major plant invaders in Spain to estimate and map their potential distribution range. We found a positive relationship between present and potential distribution of species. Most of the species have not yet occupied half of their potential distribution range. Sorghum halepense and Amaranthus retroflexus have the widest potential distribution range. Sorghum halepense and Robinia pseudoacacia have the highest relative occupancy (i.e. proportion of potential distribution range currently occupied). Species with a larger minimum residence time have, on average, higher relative occupancy. Our study warns managers that it might be only a matter of time that currently localized invasive species reach their potential area of distribution.
alien plants, climate, distribution range, landscape, minimum residence time, niche models, propagule pressure, range size, species occupancy
Invasive plant species are defined as alien species that sustain self-replacing populations without direct human intervention. They produce offspring, often in very large numbers, at considerable distances from the parent plants, and thus have the potential to spread over a large area (
Up to now, most efforts to evaluate the success of invasive species at the regional scale have been traditionally measured as the present distribution range in the region of introduction (
Moreover, historical factors determining differences in propagule pressure such as the minimum residence time (i.e. time since first record) also influence the range size of invaders (
Here we calculated and mapped the potential distribution ranges of the main invasive plant species in Spain using climatic, geographic and land use variables. This research is planned to assist environmental managers to estimate the risk of present alien invasive species to expand into non-invaded areas. Mapping the potential distribution of species in areas were they are still not occurring is of high priority for the regional administrations to fulfil the Spanish List and Catalogue of Invasive Exotic Species Act 1628/2011 (http://www.boe.es/boe/dias/2011/12/12/pdfs/BOE-A-2011-19398.pdf). Our main questions are: (i) To what extent is the potential distribution range related to the present distribution range? (ii) What is the mean proportion of potential distribution range currently occupied (i.e. relative occupancy)? (iii) Does relative occupancy depend on the minimum residence time of the species?
Materials and methods Species distributionDistribution data and minimum residence time (i.e. earliest date on which a given species was recorded in Spain) were compiled from the Atlas of Invasive Plant Species in Spain (
Environmental data were obtained from different data sources that were originally at different resolutions, but we aggregated each one of them to a 10x10 km UTM grid cell scale by averaging. All the GIS procedures involving the set up of the environmental variables were performed using MiraMon (
The selection of environmental variables was based on preliminary results on variables strongly related to invasive plant species richness in Spain (
Initial set of environmental predictors to estimate potential distribution ranges of 78 invasive plant species in Spain. Landscape variables were reduced from 10 to 5 using a principal component analysis (PCA) and keeping the first five orthogonal axes (cumulated explained variance = 80%). Distance to the coastline and 3 climatic variables were also selected. For distance to the coastline we used the residuals from the regression with annual temperature range as predictor variable. For summer rainfall, we used the residuals from multiple regressions with annual temperature range and minimum winter temperature as predictor variables (for more details see
| Variables | Data source | Transformation |
|---|---|---|
| Landscape | ||
| Built-up areas (%) | CORINE Land Cover Map of Spain (http://www.fomento.es) | PCA |
| Agricultural areas (%) | PCA | |
| Forests (%) | PCA | |
| Scrub and herbaceous associations (%) | PCA | |
| Open spaces (%) | PCA | |
| Wetlands (%) | PCA | |
| Water bodies (%) | PCA | |
| Land cover diversity (Shannon Index) | PCA | |
| Roads length (m) | Official server of the Spanish Ministry (http://www.cnig.es) | PCA |
| Railway length (m) | PCA | |
| Geography | ||
| Mean distance to the coastline (m) | Digital Elevations Model (http://www.opengis.uab.es) | Residuals |
| Climate | ||
| Annual temperature range (max July - min January) | Digital Climatic Atlas of Spain (http://opengis.uab.es/wms/iberia/index.htm) | Non transformed |
| Minimum winter temperature (°C) | Non transformed | |
| Summer rainfall (mm) | Residuals | |
In Spain, distance to coastline encompasses a complex gradient. Coastal areas concentrate the tourism, trading and transport centres, as most of the first records of alien species (
Because a precise native distribution was not known for most of the species selected, we estimated the potential range of each species using climatic, geographic and landscape information from their present distribution in Spain (see
Considering that our goal was to estimate and map the potential distribution of 78 invasive species, it was impossible to find good climatic data from the native range for all species. However, notice that we did not solely base our analysis on climatic data but also on geographic and landscape data. These variables account for habitat invasibility and propagule pressure influencing on the degree of invasion. Therefore, even if possibly our models might be climatically conservative they included other relevant landscape variables known to influence the degree of invasion (
The potential distribution range of each species was modelled as a function of the 9 selected environmental variables. All the modelling process was performed using the BIOMOD application implemented under R software. We calibrated 4 models usually described as the most powerful approaches available (
To avoid the usual trouble of selecting a particular model, we performed a weighted averaging procedure across our four models as recommended by
We analysed the relative occupancy as a function of minimum residence time. Relative occupancy was calculated as the proportion of potential distribution range currently occupied by each species. Relative occupancy was expressed as a binary variable with the first column containing the number of UTM cells currently occupied and the second column with the number of potentially suitable UTM cells not yet occupied. Minimum residence time was log transformed before analysis to meet the assumptions of parametric analysis.
Invasive species are a non-random subset of all species introduced (
Analyses were conducted in the open source R software version 2.5.1 (R Development Core Team 2005). We modelled relative occupancy with a binomial distribution of errors using the glmmPQL of the MASS library on the R statistical package (
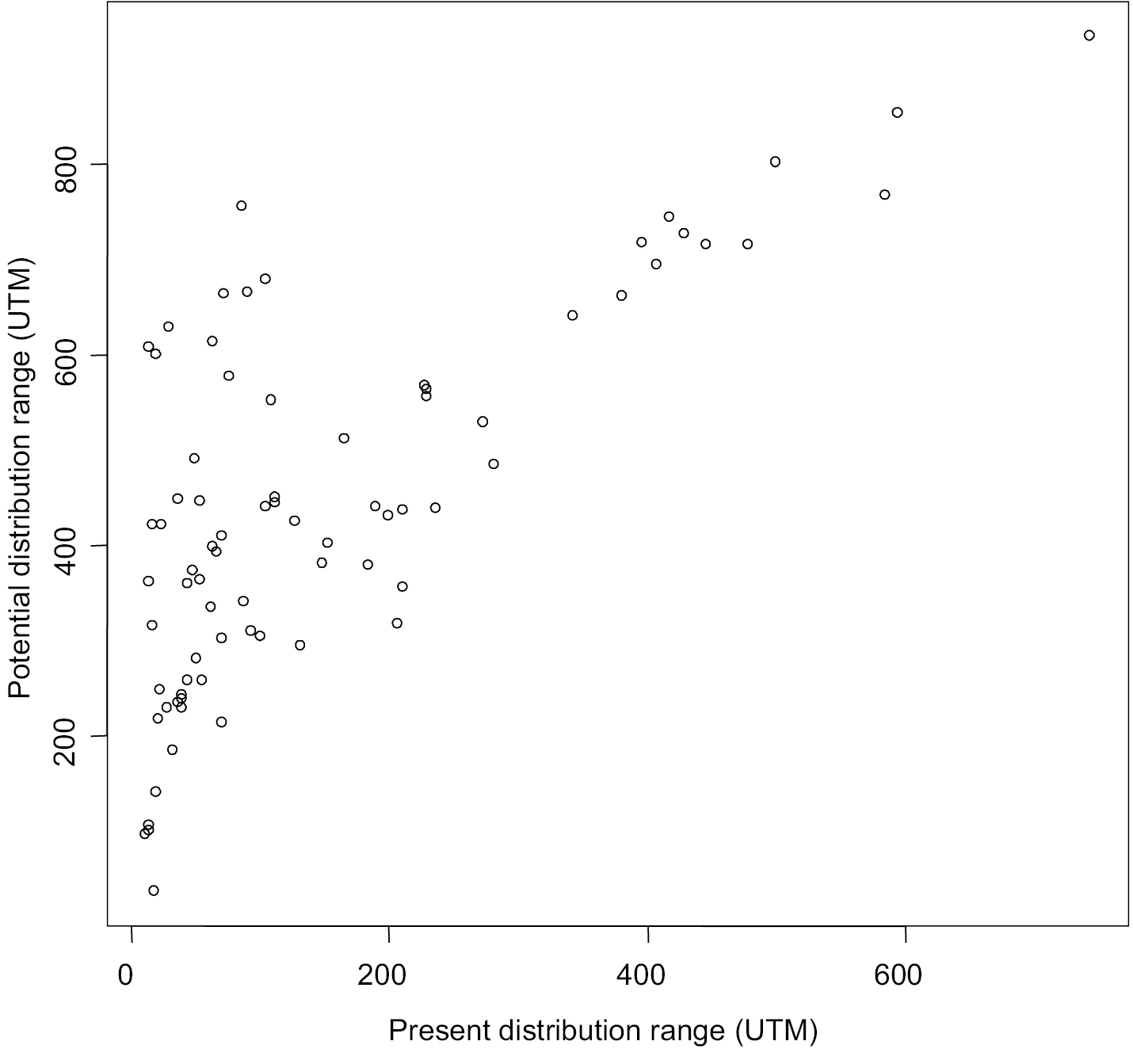
We are confident that our models to estimate the potential distribution range were very good because for most species AUC>0.9 (Appendix I). As expected, there was a positive relationship between present distribution ranges (CDR) and potential distribution ranges (PDR). However, present distribution ranges only explained half of the variance of the potential distribution ranges (PDR = 309.3 + 0.89CDR, R2 = 0.53, F(1, 76) = 85.44, p < 0.01). There was a set of species, especially those that currently occupy less than 200 UTM that, according to our models, would have the potential to spread through larger areas than that expected by the linear relationship (Fig. 1).
Relationship between present (CDR) and potential (PDR) distribution ranges for 78 invasive plant species in Spain. PDR = 309.3 + 0.89CDR, R2 = 0.53, F(1, 76) = 85.44, p < 0.01.
The species with the widest potential distribution ranges were Sorghum halepense, considered as one of the top weeds in the world (
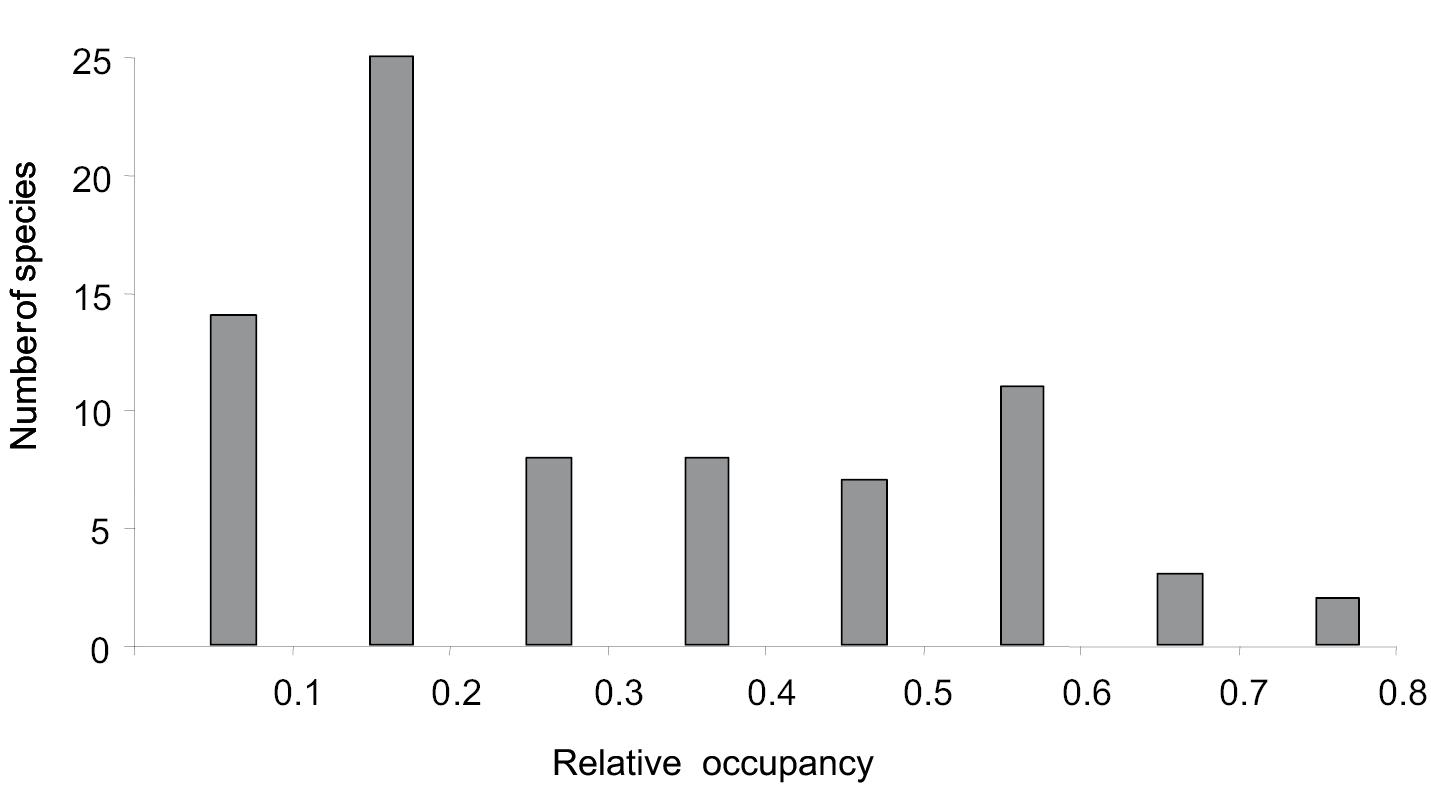
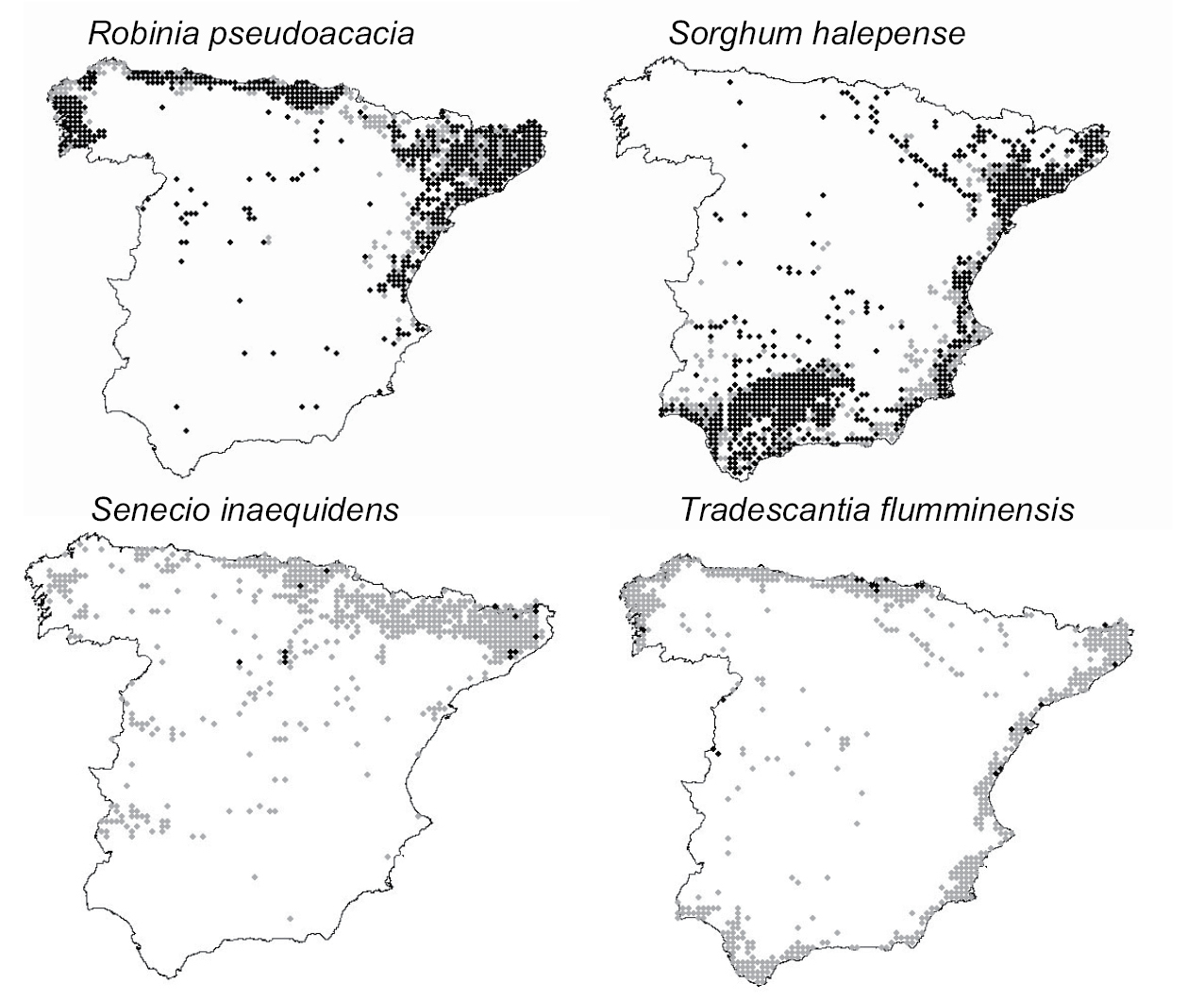
The mean (±SE) relative occupancy of species was 0.28 ± 0.02; with values ranging from 0.05 to 0.73. Most of the species have not yet occupied half of their potential geographic ranges (Fig. 2). The two species with the lowest relative occupancy were the shrub Senecio inaequidens (0.28) and the herb Tradescantia flumminensis (0.23), and the two species with the highest relative occupancy were the deciduous tree Robinia pseudoacacia (0.73) and the grass Sorghum halepense (0.72) (Fig. 3).
Frequency distribution of relative occupancy (i.e. proportion of the potential distribution range currently occupied) by invasive plant species in Spain (N = 78).
Maps of present distribution range (black) over potential distribution range (grey) of four invasive plant species in Spain. The two species on top are the ones with the highest relative occupancy (i.e. proportion of the potential distribution range currently occupied) and the ones at the bottom are those with the lowest. Maps for the remaining analysed 74 invasive species are available in Appendix II.
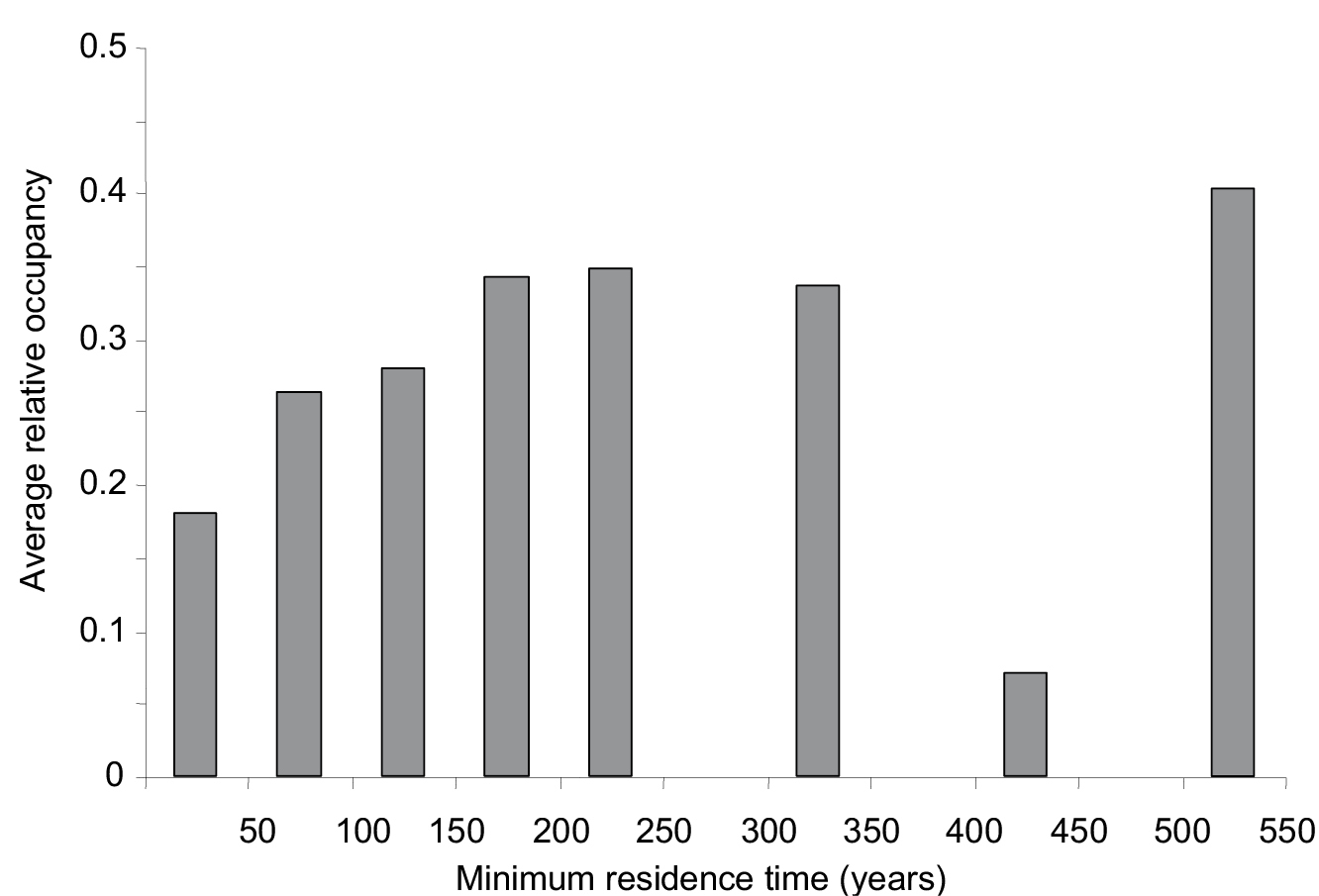
Having accounted for the potential phylogenetic effects, the glmmPQL showed that minimum residence time explained a significant portion of variance in relative occupancy (t = 3.9, p<0.0001). Species introduced earlier had, on average, occupied a higher proportion of their potential distribution range (Fig. 4). However, it is interesting to note that the relationship is not linear, with some species introduced a long time ago (i.e. 400–450 years) having still a very restricted distribution with respect to their modelled potential distribution range.
Average relative occupancy (i.e. proportion of the potential distribution range currently occupied) for minimum residence time classes of invader plant species in Spain (N = 78).
We found large differences among species in their potential distribution range in Spain. However, in general, invasive species have not yet reached half of their potential distribution ranges. On average, it takes around 150 years for a neophyte to reach its maximum distribution range in an European country (
Our calculations of potential distribution ranges for the 78 invasive species are based on climatic conditions in the introduced range in Spain and not in the native range. Theoretically, modelling the potential distribution range of an alien species should be based on climate matching envelopes build with information from the native range where the species is at equilibrium (
Results confirmed that relative occupancy was dependent on minimum residence time (
Similarly, the association between relative occupancy and minimum residence time is weak for species introduced many centuries ago (
Besides propagule pressure, differences in the potential distribution range might be also explained by differences in their niche breadth (
Our study has been possible because there was reliable spatially explicit data on the present distribution of invasive species (
We thank F. De Bello, I. Ibáñez, I. Kühn, S. Lavergne, P. Pyšek and three anonymous referees for comments on an early version of the manuscript. This study has been partially financed by ALARM (GOCE-CT-2003-506675) and DAISIE (SSPI-CT-2003-511202) within the 6th FP of the EU; the Spanish Ministerio de Ciencia e Innovación projects RIXFUTUR (CGL2009-7515) and MONTES (CSD2008-00040); the Junta de Andalucía project RNM-4031; and the ANR SCION project (ANR-08-PEXT-03). This paper contributes to the forthcoming Virtual Lab on Biological Invasions within the LIFEWATCH framework.
Minimum residence time (MRT; years), present distribution ranges (CDR; number of UTM 10x10 km), potential distribution ranges (PDR; number of UTM 10×10 km) and relative occupancy (i.e. proportion of the potential distribution range currently occupied) for 78 invasive plant species in Spain. PDR were calculated according to an averaged model of 4 potential distribution models for each species. The area under the ROC curve (AUC) of the averaged model is shown on the table.
| Family | Species | MRT | CDR | PDR | OCCUPANCY | AUC |
|---|---|---|---|---|---|---|
| Malvaceae | Abutilon theophrasti | 74 | 94 | 680 | 0.14 | 0.92 |
| Fabaceae | Acacia cyanophylla | 204 | 20 | 217 | 0.09 | 0.98 |
| Fabaceae | Acacia dealbata | 204 | 204 | 356 | 0.57 | 0.99 |
| Fabaceae | Acacia melanoxylon | 204 | 71 | 214 | 0.33 | 1.00 |
| Aceraceae | Acer negundo | 204 | 61 | 393 | 0.16 | 0.95 |
| Asteraceae | Achillea filipendulita | 22 | 18 | 37 | 0.49 | 1.00 |
| Agavaceae | Agave americana | 504 | 217 | 557 | 0.39 | 0.97 |
| Simaroubaceae | Ailanthus altísima | 186 | 210 | 563 | 0.37 | 0.96 |
| Amaranthaceae | Amaranthus albus | 143 | 408 | 716 | 0.57 | 0.95 |
| Amaranthaceae | Amaranthus blitoides | 94 | 445 | 715 | 0.62 | 0.96 |
| Amaranthaceae | Amaranthus hybridus | 124 | 368 | 718 | 0.51 | 0.94 |
| Amaranthaceae | Amaranthus muricatus | 96 | 189 | 432 | 0.44 | 0.97 |
| Amaranthaceae | Amaranthus powellii | 21 | 83 | 666 | 0.12 | 0.93 |
| Amaranthaceae | Amaranthus retroflexus | 104 | 551 | 854 | 0.65 | 0.94 |
| Amaranthaceae | Amaranthus viridis | 106 | 100 | 444 | 0.23 | 0.95 |
| Asclepiadaceae | Araujia sericifera | 28 | 90 | 311 | 0.29 | 0.98 |
| Asteraceae | Arctotheca calendula | 88 | 23 | 421 | 0.05 | 0.97 |
| Asteraceae | Artemisia verlotiorum | 91 | 158 | 512 | 0.31 | 0.97 |
| Asteraceae | Aster squamatus | 92 | 320 | 641 | 0.50 | 0.96 |
| Cactaceae | Austrocylindropuntia subulata | 20 | 48 | 282 | 0.17 | 0.98 |
| Asteraceae | Baccharis halimifolia | 55 | 14 | 106 | 0.13 | 1.00 |
| Asteraceae | Bidens aurea | 41 | 64 | 663 | 0.10 | 0.91 |
| Asteraceae | Bidens frondosa | 60 | 83 | 341 | 0.24 | 0.97 |
| Asteraceae | Bidens pilosa | 91 | 21 | 249 | 0.08 | 0.98 |
| Asteraceae | Bidens subalternans | 69 | 131 | 295 | 0.44 | 0.99 |
| Poaceae | Bromus willdenowii | 100 | 144 | 403 | 0.36 | 0.97 |
| Buddlejaceae | Buddleja davidii | 43 | 93 | 305 | 0.30 | 0.98 |
| Aizoaceae | Carpobrotus edulis | 104 | 98 | 441 | 0.22 | 0.97 |
| Poaceae | Chloris gayana | 102 | 23 | 249 | 0.09 | 0.99 |
| Asteraceae | Conyza bonariensis | 132 | 348 | 662 | 0.53 | 0.95 |
| Asteraceae | Conyza canadensis | 220 | 456 | 803 | 0.57 | 0.94 |
| Asteraceae | Conyza sumatrensis | 100 | 265 | 485 | 0.55 | 0.96 |
| Poaceae | Cortaderia selloana | 35 | 61 | 335 | 0.18 | 0.98 |
| Asteraceae | Cotula coronopifolia | 107 | 42 | 361 | 0.12 | 0.97 |
| Solanaceae | Datura innoxia | 504 | 62 | 410 | 0.15 | 0.94 |
| Solanaceae | Datura stramonium | 504 | 386 | 744 | 0.52 | 0.95 |
| Poaceae | Echinochloa hispidula | 36 | 37 | 242 | 0.15 | 0.99 |
| Poaceae | Echinochloa oryzicola | 104 | 36 | 449 | 0.08 | 0.98 |
| Poaceae | Echinochloa oryzoides | 36 | 28 | 629 | 0.04 | 0.95 |
| Elaeagnaceae | Elaeagnus angustifolia | 204 | 54 | 614 | 0.09 | 0.92 |
| Poaceae | Eleusine indica | 143 | 43 | 259 | 0.17 | 0.99 |
| Hydrocharitaceae | Elodea canadensis | 99 | 13 | 362 | 0.04 | 0.97 |
| Myrtaceae | Eucalyptus camaldulensis | 204 | 70 | 578 | 0.12 | 0.95 |
| Myrtaceae | Eucalyptus globulus | 204 | 200 | 317 | 0.63 | 0.99 |
| Polygonaceae | Fallopia baldschuanica | 26 | 79 | 757 | 0.10 | 0.94 |
| Caesalpiniaceae | Gleditsia triacanthos | 56 | 51 | 364 | 0.14 | 0.97 |
| Asclepiadaceae | Gomphocarpus fruticosus | 242 | 53 | 259 | 0.20 | 0.99 |
| Boraginaceae | Heliotropium curassavicum | 130 | 36 | 230 | 0.16 | 0.98 |
| Asteraceae | Helianthus tuberosus | 304 | 179 | 380 | 0.47 | 0.98 |
| Convolvulaceae | Ipomoea indica | 104 | 144 | 381 | 0.38 | 0.98 |
| Convolvulaceae | Ipomoea purpurea | 104 | 101 | 553 | 0.18 | 0.94 |
| Convolvulaceae | Ipomoea sagittata | 304 | 36 | 235 | 0.15 | 0.99 |
| Verbenaceae | Lippia filiformis | 404 | 44 | 490 | 0.09 | 0.95 |
| Caprifoliaceae | Lonicera japonica | 43 | 70 | 303 | 0.23 | 0.99 |
| Nyctaginaceae | Mirabilis jalapa | 119 | 223 | 438 | 0.51 | 0.98 |
| Solanaceae | Nicotiana glauca | 152 | 120 | 425 | 0.28 | 0.96 |
| Onagraceae | Oenothera biennis | 156 | 100 | 451 | 0.22 | 0.96 |
| Onagraceae | Oenothera glazioviana | 80 | 50 | 446 | 0.11 | 0.95 |
| Cactaceae | Opuntia dillenii | 130 | 17 | 421 | 0.04 | 0.99 |
| Cactaceae | Opuntia ficus-indica | 504 | 396 | 728 | 0.54 | 0.96 |
| Oxalidaceae | Oxalis pes-caprae | 154 | 199 | 437 | 0.46 | 0.97 |
| Poaceae | Paspalum dilatatum | 97 | 178 | 441 | 0.40 | 0.97 |
| Poaceae | Paspalum paspalodes | 180 | 216 | 568 | 0.38 | 0.97 |
| Poaceae | Paspalum vaginatum | 97 | 38 | 239 | 0.16 | 0.98 |
| Polygonaceae | Reynoutria japonica | 30 | 14 | 100 | 0.14 | 1.00 |
| Fabaceae | Robinia pseudoacacia | 304 | 559 | 768 | 0.73 | 0.97 |
| Asteraceae | Senecio inaequidens | 20 | 14 | 609 | 0.02 | 0.94 |
| Asteraceae | Senecio mikanioides | 71 | 19 | 141 | 0.13 | 0.96 |
| Solanaceae | Solanum bonariense | 121 | 42 | 374 | 0.11 | 0.97 |
| Fabaceae | Sophora japonica | 304 | 11 | 97 | 0.11 | 1.00 |
| Poaceae | Sorghum halepense | 204 | 674 | 935 | 0.72 | 0.95 |
| Poaceae | Spartina patens | 87 | 32 | 186 | 0.17 | 1.00 |
| Poaceae | Stenotaphrum secundatum | 101 | 27 | 230 | 0.12 | 0.97 |
| Commelinaceae | Tradescantia fluminensis | 304 | 17 | 601 | 0.03 | 0.92 |
| Tropaeolaceae | Tropaeolum majus | 404 | 16 | 315 | 0.05 | 0.97 |
| Asteraceae | Xanthium spinosum | 304 | 367 | 694 | 0.53 | 0.95 |
| Asteraceae | Xanthium strumarium | 122 | 262 | 530 | 0.49 | 0.97 |
| Zygophyllaceae | Zygophyllum fabago | 143 | 58 | 399 | 0.15 | 0.96 |
Maps of present distribution range over potential distribution range for 78 invasive plant species in Spain. (doi: 10.3897/neobiota.12.2341.app2) File format: PDF.
Explanation note: Maps of present distribution range (purple) over potential distribution range (grey) for 78 invasive plant species in Spain. Potential distribution ranges were calculated by niche models as a function of climatic, geographic and landscape variables on the present distribution of species at 10×10 km UTM resolution. Niche models were build by weighted averaging procedures across GLM, GAM, RF and GBM models.
Copyright notice: This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.
Citation: Gassó N, Thuiller W, Pino J, Vilà M (2012) Potential distribution range of invasive plant species in Spain. NeoBiota 12: 25–40. doi: 10.3897/neobiota.12.2341.app2