(C) 2012 Leonard A. Freed. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Many successful invasions involve long initial periods in which the invader exists at low densities followed by sudden population increases. The reasons for such time-lags remain poorly understood. Here we document a sudden increase in density of the introduced Japanese white-eye (Zosterops japonicus) in a restoration area contiguous with old-growth forest at Hakalau Forest National Wildlife Refuge on the Island of Hawaii. The refuge, with very high density of native birds, existed in a pocket of low white-eye density that persisted for at least 20 years since the late 1970s. The refuge began an extensive native trees restoration project in 1989 within a 1314 ha abandoned pasture above old-growth forest. This area was soon colonized by white-eyes and their population grew exponentially once the trees had grown tall enough to develop a canopy. This increase was in turn followed by significantly more white-eyes in the open and closed forests adjacent to the restoration area. Competition between white-eyes and native species was documented on study sites within these forests. Density data indicate that competition was more widespread, with loss of tens of thousands of native birds in the 5371 ha area surveyed. Our results are consistent with the view that ecological barriers may delay the population increase of invaders and that human-derived activities may help invaders cross these barriers by creating new ecological opportunities. Control of white-eye numbers may be essential for recovery of native species.
Introduced bird, restoration, exponential population growth, propagule pressure, biotic resistance, competition, time-lag
When introduced into novel environments, some invaders increase rapidly in the new location, while others experience time-lags of various lengths (
Restoration is a human-derived environmental change that is known to provide ecological opportunities in habitat for plants and animals (
This paper focuses on the invasion of Hakalau Forest National Wildlife Refuge (Mauna Kea, Island of Hawaii) by the Japanese white-eye (Zosterops japonicus), a passerine bird native to Asia. The white-eye was intentionally introduced to the Island of Oahu in 1929 to control insects (
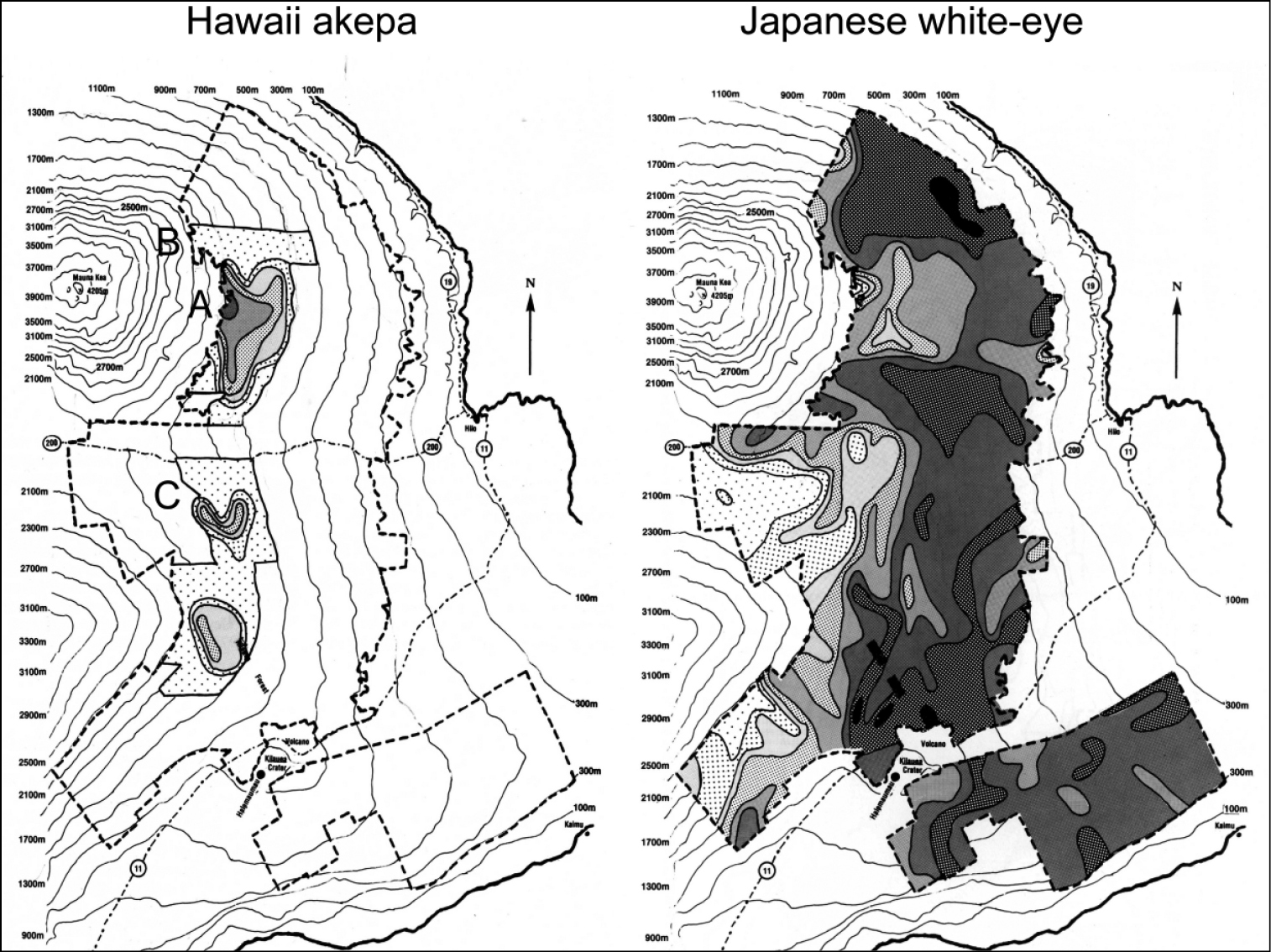
Nevertheless, on Mauna Kea, in the land that eventually became Hakalau Forest National Wildlife Refuge in 1985, the white-eye remained at low densities documented in the late 1970s. The refuge has the highest density of native birds on the island (
Our goal is to describe how the refuge restoration area brought a sudden increase in white-eye density, and to ask whether this sudden increase may explain the recent increase of the invader in adjacent open and closed forests with native birds. Documenting the increased density of white-eyes throughout the refuge is particularly relevant given the diverse evidence that the species is negatively affecting native birds (
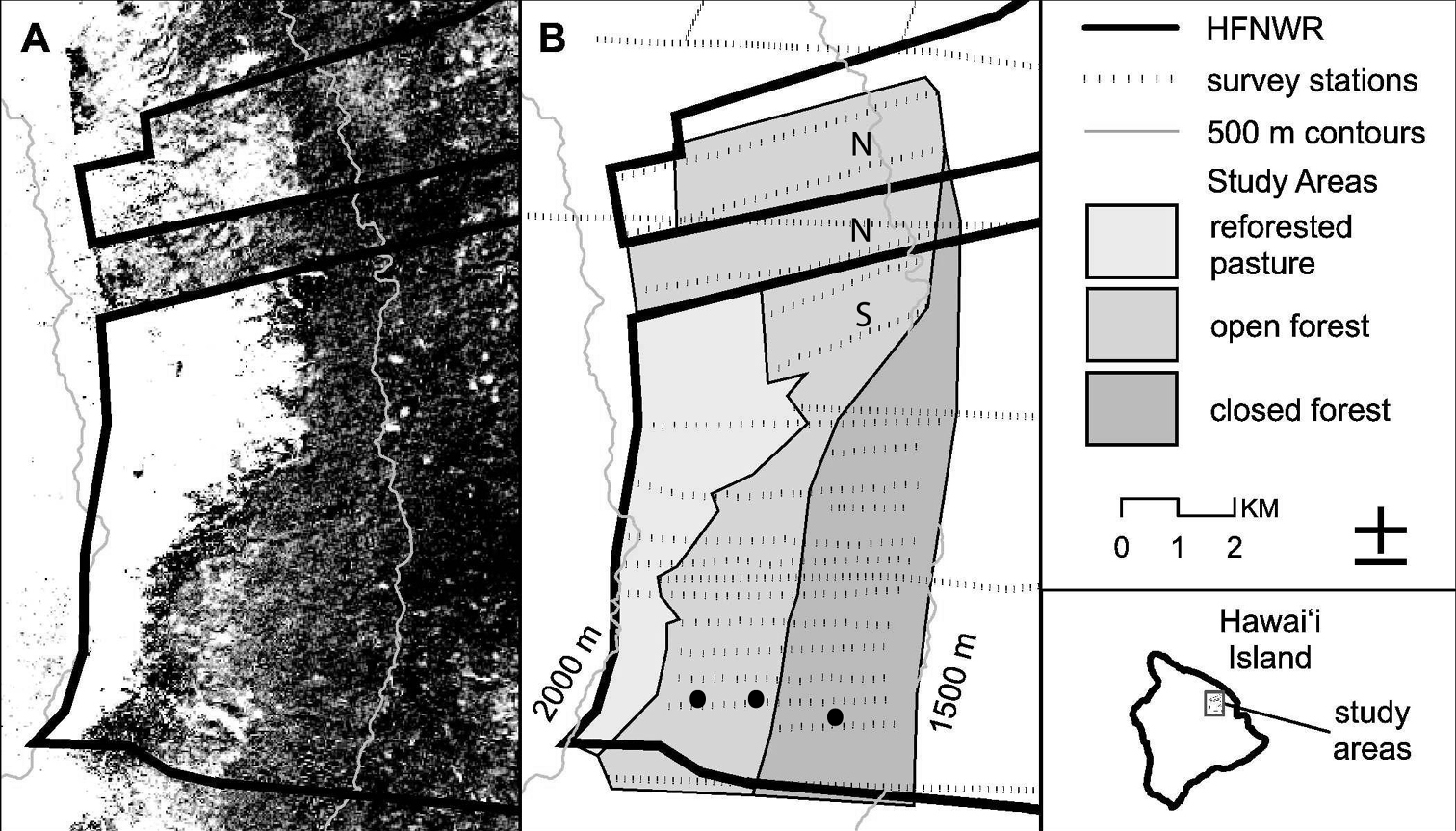
Hakalau Forest National Wildlife Refuge (Fig. 1) consists mainly of old-growth ohia-lehua/koa (Metrosideros polymorpha/Acacia koa) forest (
Maps of distribution and abundance of the endangered Hawaii akepa (Loxops coccineus coccineus) (left) and introduced Japanese white-eye (right) in the windward Hawaii study area from the Hawaii Forest Bird Survey conducted in the late 1970s (
Portion of Hakalau Forest National Wildlife Refuge that was surveyed, relative to aerial photograph A and configuration of restoration, open forest, and closed forest areas B based on
Estimates of white-eye density and variance from survey data were obtained from
For the open forest area, we also used a randomization test for a stepwise increase during 1988–1999 and 2000–2007. The time periods were based on mist-netting that indicated a stepwise increase beginning in 2000 (
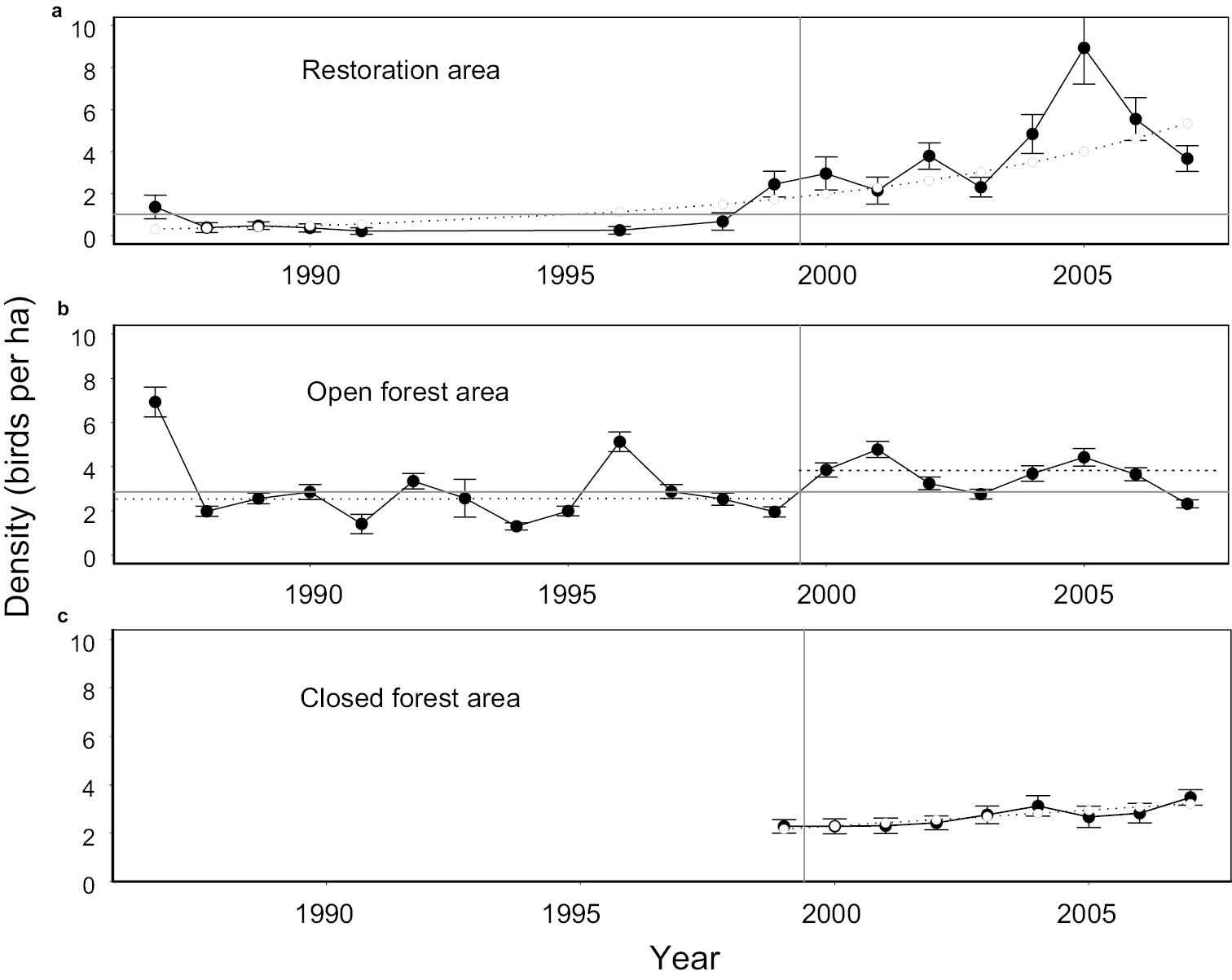
Long-term patterns of Japanese white-eye density with standard error in: a restoration area b open forest area, and c closed forest area. Fitted values from the best model (deviation) are shown in a) and c). Median of the entire series is shown in a) and b). For b), medians for 1987–1999 and for 2000–2007 are shown in dotted lines.
We determined the order of increase between areas in two ways. For the restoration area and the open forest area, we compared slopes of regressions of density on year for 1988–2000. This time period was selected because it occurred before the stepwise increase from mist-netting. A significant slope in the restoration area but not in the open forest area would establish order. For the open forest area and closed forest area, we compared sequential differences in density from 1999 to 2000 and to 2001 in relation to the standard errors of the estimates. We determined if the differences between years were greater than two standard errors of those differences, indicating growth, or no growth if the differences were less than two standard errors.
We use functional data analysis (
This function tests the same hypothesis as regression, change over time, and it is particularly relevant for documenting the consequences of an environmental change. Unlike regression, the functional data analysis is not constrained by constant change per unit time, or by the location of the intercept relative to the data. There is no theory in population dynamics that predicts constant rate of change. The analysis can also estimate the magnitude of change using basic mathematical properties of mean and variance of sums and differences of random variables (
The two endpoints we compare are densities in 2000 and 2007. The year 2000 is the first year of an environmental change (
We illustrate with this method the decline in the open forest area of the iiwi (Vestiaria coccinea), currently a species of concern in the process of being listed as endangered. The density in 2000 was 23.80 birds/ha, bracketed by 19.59 in 1999 and by 17.02 in 2001, for a mean density of 20.14. The density in 2007 was 7.77 birds/ha bracketed by 15.92 in 2006, for a mean density of 11.85. The standard deviations were 0.0641, 0.890, and 0.722 for years 1999–2001 and 0.0766 and 0.0634 for years 2006–2007. Squaring these gives the variances.Then, the sum of variances for each set of years is calculated using the following property of variance of the sum of independent random variables: Var(a1X1 + ... + anXn) = a12Var(X1) + ... + an2Var(Xn), where the ai are coefficients. The mean is the sum of random variables with the ai equal to 1/n, where n is the sample size. For the iiwi, the means are the sum of independent random variables X1/3 + X2/3 + X3/3 for 1999–2001 and X1/2 + X2/2 for 2006–2007. Therefore the variance of the mean random variables are (0.0041 + 0.7921 + 0.5213)/9 = 0.1464 for 2000 and (0.0059 + 0.0040)/4 = 0.0002 for 2007. Then, the difference between the means, 20.14 - 11.85 = 8.29 becomes a new random variable representing the decline. The variance of the difference between independent random variables is the same as the variance of the sum. Thus the variance of the difference is 0.1464 + 0.0002 = 0.1466. Taking the square root gives the standard error of the difference as 0.3829. Twice the standard error on either side of the 8.29 birds/ha difference between 2000 and 2007 gives the 95% confidence interval of (7.52, 9.06). Because the confidence interval does not include 0, we can conclude that a significant decline has occurred in the open forest area. The extent of the decline can be estimated by multiplying the 8.29 birds/ha change by the 3373 ha area.
We use this same methodology to document the white-eye increase in the closed forest area. For the open forest area, we use the difference in means established by the randomization test, and then calculate the variances associated with the standard deviations from all years during 1988–1999 and 2000–2007. Then the 95% confidence interval was calculated as specified for 2000 and 2007.
For community-wide changes in each survey area, we took covariance among species into account. We used the variance of densities of all 8 native species during 2000–2007 plus the covariance of densities from all pairs of native species those same years, according to the standard statistical formula Var(X+Y) = Var(X) + Var(Y) + 2Cov(X, Y), extended to 8 species. From this total, the 95% confidence interval of variances of the difference in density in the community was calculated.
ResultsRestoration area. White-eyes increased in the restoration area, especially from 1999 to 2007 (Fig. 3a). They had low or 0 density when seedlings were first planted in 1989, and were mainly present in the few remnant trees along predominantly dry streams following the mountain slope in the area. The linear growth model for the entire series was significant (slope = 0.27 ± 0.06, R2 = 0.57, p = 0.0007), but was dismissed because it had significant lack of fit. This was indicated by a run of 7 negative residuals (sign test, p = 0.01). The exponential growth model for the entire series was also significant (parameter = 0.14 ± 0.03, R2 = 0.66, p = 0.0001), with sufficient fit indicated by runs of no more than 4 positive or negative residuals. The increase did not really start until 1998 (Fig. 3a).
Open forest area. White-eyes also increased in the open forest area where they previously existed at lower density (Fig. 3b). Linear regression did not reveal the increase (slope = 0.01 ± 0.05, R2 = 0.004, p = 0.78). However, residuals had significant lack of fit, indicated by 2/13 positive residuals during 1987–1999 and 6/8 positive residuals during 2000–2007 (test of proportions, p = 0.023). The exponential regression model had adequate fit (runs of maximum length 3) but was not significant (parameter = 0.01 ± 0.02, R2 = 0.035, p = 0.49). Nevertheless, the randomization test clearly indicated a higher mean density during years 2000–2007 (critical value for 0.975 quantile = 0.97, observed difference = 1.05), consistent with a stepwise increase that could not be modeled with linear or exponential regression.
Closed forest area. White-eye density also increased in the closed forest area, where the birds had previously existed at low density (Fig. 3c). Both linear and exponential models were supported (linear: slope = 0.13 ± 0.03, R2 = 0.74, p = 0.003; exponential: parameter = 0.05 ± 0.01, R2 = 0.77, p = 0.002), had adequate fit by runs of signed residuals, and were indistinguishable (ΔAICc exponential = 0, linear = 0.16).
Order of increase among areas. During years 1988–2000, density increased in the restoration area (slope = 0.6, se = 0.06, p = 0.04), but not the open forest area (slope = 0.08, se = 0.08, p = 0.33). Thus the increase began in the restoration area before the open forest area. Changes in density during the years 1999–2001 were significant in the open forest area (1.90 ± 0.79, 2.82 ± 0.85), but not in the closed forest area (0.01 ± 0.42, 0.03 ± 0.85). The overall order of increase was from the restoration to the open forest to the closed forest area (Fig. 3).
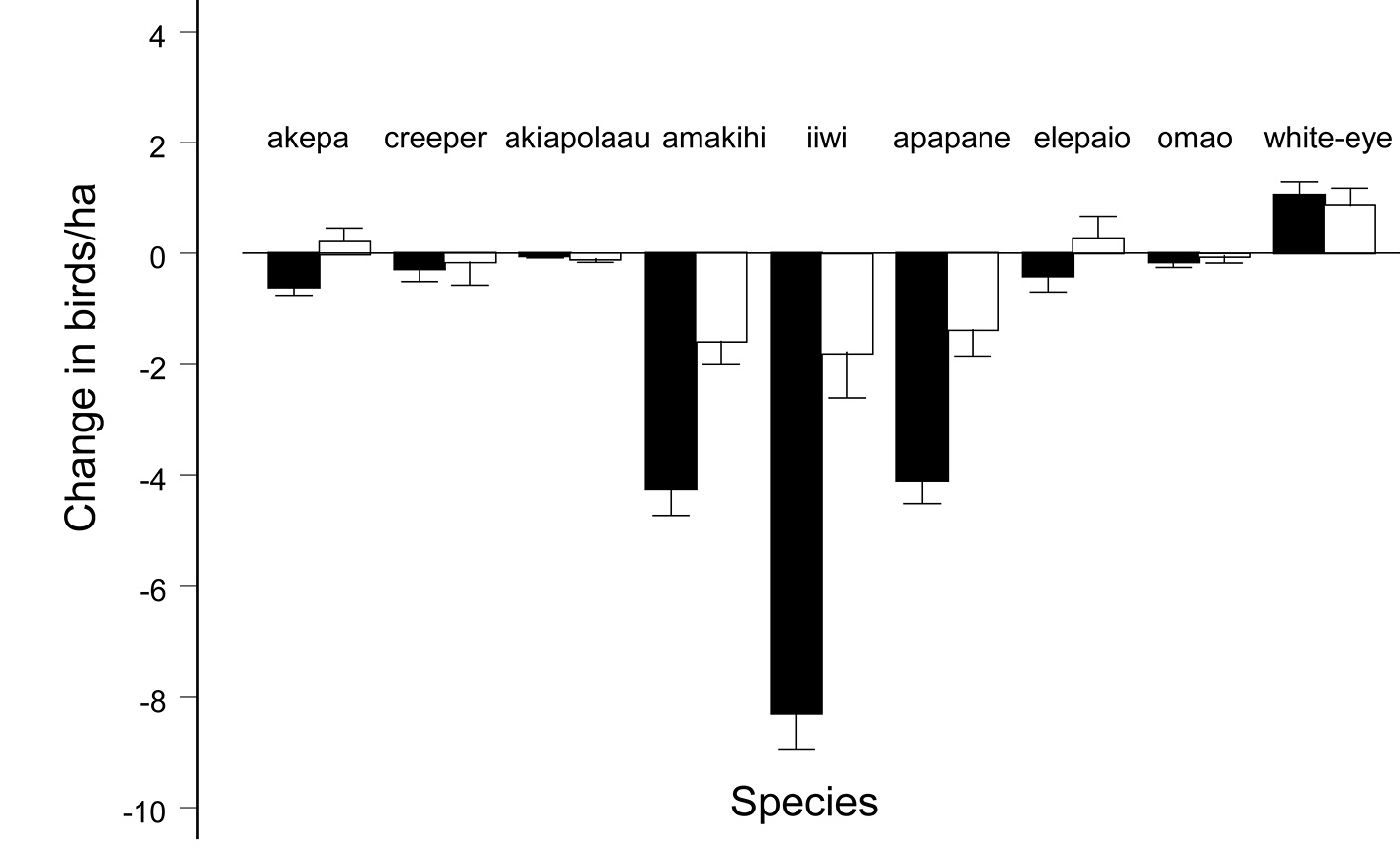
Changes in native birds in the forested areas. Figure 4 shows significant declines in native birds the same years that the white-eye was increasing in both the open and closed forest areas. The declines in the closed forest area were smaller than those estimated in the open forest area (Fig. 4, paired t = 2.15, df =7, p = 0.034). However, the high correlation between changes in density in the two forest areas (cor = 0.93, p = 0.0003), including white-eyes, indicates that the pattern of changes among native species and white-eyes was similar in the open and closed forest areas. Table 1 shows that tens of thousands of native birds perished even under the most optimistic scenario. Based on mean 2000 densities times 3373 ha, there were approximately 166, 927 native birds in the open forest area. The loss of 61, 289 birds represents a 37% decline, ranging from 51 to 22%. The closed forest area had 86, 653 birds with a loss of 9091, representing a 10% decline. The white-eye was the only bird that significantly increased in each area (Fig. 4, Table 1).
Change in densities with standard error of native birds and Japanese white-eyes in the open forest area (black bars) and closed forest area (white bars), between 2000 and 2007. Native passerine species present throughout the closed forest area and the southern section of the open forest area were Hawaiian honeycreepers: Hawaii akepa (Loxops coccineus coccineus), Hawaii creeper (Oreomystis mana), akiapolaau (Hemignathus munroi), Hawaii amakihi (Hemignathus virens virens), iiwi (Vestiaria coccinea), and apapane (Himatione sanguinea); Monarchine flycatcher: Hawaii elepaio (Chasiempis sandwichensis ridgwayi); and Thrush: omao (Myadestes obscurus).
Changes in numbers between 1999–2001 and 2006–2007 in open and closed forest (Fig. 1), based on changes in density multiplied by area (ha). Endangered species (E), species of concern (SOC). Confidence intervals for species are based on two standard errors of changes in density. Confidence intervals for community change are based on two standard errors of the sum of variances and twice the sum of covariances of all native species densities from 2000 through 2007.
| Species | Change in Numbers | Lower 95% CI | Upper 95% CI |
|---|---|---|---|
| Open Forest | |||
| Hawaii akepa (E) | -2, 081 | -3, 080 | -1, 083 |
| Hawaii creeper (E) | -968 | -2, 506 | 570 |
| Akiapolaau (E) | -169 | -405 | 69 |
| Hawaii amakihi | -14, 329 | -17, 587 | -11, 070 |
| Iiwi (SOC) | -27, 969 | -32, 442 | -23, 496 |
| Apapane | -13, 823 | -16, 602 | -11, 043 |
| Hawaii elepaio | -1, 400 | -3, 329 | 530 |
| Omao | -550 | -1, 191 | 91 |
| Community change: | -61, 289 | -85, 892 | -36, 686 |
| Japanese white-eye | 3, 542 | 5, 083 | 2, 001 |
| Closed Forest | |||
| Hawaii. akepa (E) | 440 | -515 | 1, 395 |
| Hawaii creeper (E) | -320 | -1, 990 | 1, 351 |
| Akiapolaau (E) | -180 | -432 | 72 |
| Hawaii amakihi | -3, 177 | -10, 557 | -7, 305 |
| Iiwi (SOC) | -3, 576 | -6, 833 | -320 |
| Apapane | -2, 737 | -4, 707 | -767 |
| Hawaii elepaio | 539 | -1, 083 | 2, 162 |
| Omao | -80 | -615 | 456 |
| Community change: | -9, 091 | -12, 778 | -5, 404 |
| Japanese white-eye | 1, 738 | 2, 829 | 647 |
Land that became Hakalau Forest National Wildlife Refuge existed for years in a pocket of low white-eye density, especially in the southern end with the intact bird community (
Increase of white-eye numbers. The restoration is a clear example of a human activity that increases ecological opportunities for an introduced species that otherwise should have remained at low numbers. The restoration also offered opportunities for three native species (
The increase of white-eyes in the open forest area seems to be due more to immigration from the restoration area than to in situ reproduction. This is because juvenile survival dropped from 0.27 to 0 for all years during the increase (
However, the 0 juvenile survival of white-eyes was not associated with lower mass (
The relatively small magnitude of the increase in white-eye density in the open forest area may have been severely underestimated. The area is very large (3373 ha) and includes introduced forest and forest with more open structure in the northern sections (
Impacts of white-eyes on native species. Competition between species is indicated by change in condition, survival, and/or numbers of one species as the other increases or decreases in numbers (
The piecewise regression used by
In previous studies, we identified changes in condition of native birds in our open forest sites that could be produced only by a factor that influenced foraging substrates used by each native species. Changes included lower mass, lower fat, shorter legs, and shorter bills (
None of the skeletal changes in native birds in the open forest sites were observed in the closed forest site during 2004–2005, where white-eye capture rates were only 20% of open forest rates (
The impact of direct competition in the decline of native species cannot be ascertained without experimental evidence, but alternative explanations are unconvincing. Avian malaria kills native birds, but it is too cool at upper elevations for avian malaria to be endemic (
Our data also presage connections between biotic resistance, diffuse competition, and a reversal of diffuse competition through propagule pressure. Biotic resistance relevant to this system is diffuse competition, which arises when multiple native species overlap different parts of the niche of a potential invader (
The pocket of low white-eye density that persisted for decades is now filling up with white-eyes from the restoration area. That area is a complete counterexample to restoration areas constraining invasion (
The U.S. Fish and Wildlife Service, in formulating its final comprehensive conservation plan in late 2010 (http://www.fws.gov/hakalauforest/planning.html), emphasized habitat restoration efforts on the refuge. Evidence presented here indicates that this plan will provide more ecological opportunities for white-eyes, and this will likely further increase the negative impacts on native birds in the forest below. The plan assumes that avian malaria is the greatest long-term problem for the birds (
A white-eye removal experiment must be performed to determine conclusively if competition is the cause of current food limitation in native birds and extensive declines. An experiment in New Zealand identified positive responses from multiple native species when an introduced bird was controlled (
We gratefully respect the dedication of many students, volunteers, and interns who assisted with the field research. T. Smith, D. Sol, and anonymous reviewers gave useful comments on the manuscript. We were supported over the years by grants from the John D. and Catherine T. MacArthur Foundation (World Environment and Resources Program), National Science Foundation, Environmental Protection Agency (Science to Achieve Results, R82-9093), and Australian Research Council Discovery-Project Grant DP451402 to LAF (Partner Investigator; H. McCallum, Chief Investigator).