(C) 2012 Madalin Parepa. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Invasive knotweeds, native to Eastern Asia, are among the most dominant plant invaders of European and North American temperate ecosystems. Recent studies indicate that one cause of this dominance might be allelopathy, but the possible sources and modes of action of this allelopathy are insufficiently understood. Here, we asked whether the invasive knotweed Fallopia × bohemica can exert allelopathic effects on native plants also through its leaf litter, or through persistent soil contaminants, and whether these affect the germination or growth of native plants. In a germination experiment with nine native species neither litter leachate, an aqueous extract of knotweed leaves added to the soil, nor trained soil with a history of Fallopia pre-cultivation suppressed the germination or early growth of natives. A mesocosm study with experimental native communities showed that the presence of Fallopia × bohemica, although not a dominant in these communities, caused significant shifts of life-history strategy in two dominant natives, and that similar effects could be elicited through litter leachates or trained soil alone. However, there were hardly any effects on the biomass of natives. Our study indicates that knotweed allelopathy acts on the growth rather than germination of natives, and that soil contamination through persistent allelochemicals may not be a significant problem in habitat restoration. It also shows that allelopathic effects can sometimes be subtle changes in life-history and allocation patterns of the affected species.
Biological invasions, Fallopia × bohemica, litter leachate, plant-plant interactions, soil training
Explaining the success of highly invasive plants requires a solid understanding of the mechanisms by which they interfere with native competitors. Oftentimes, invasive plants are superior resource competitors (
Allelopathy is the chemically mediated interference between co-occurring plants, where secondary compounds exuded by plant roots or leaves affect the germination or growth of neighbouring plants (
One of the most aggressive and at the same time least understood group of plant invaders are the clonal knotweeds Fallopia japonica and Fallopia sachalinensis, and their hybrid Fallopia × bohemica. Originally introduced from Eastern Asia as ornamentals (
Previous studies have shown that invasive knotweeds contain several potentially allelopathic compounds (
Here, we investigated the effects of Fallopia × bohemica litter leachates and trained soil on a range of native European plant species. We carried out two experiments, one in which we tested for allelopathic effects on the germination and establishment of individual plants, and a second one in which we examined allelopathic effects on experimental communities of several native species. Specifically, we tested the following hypotheses: (1) Litter leachates of Fallopia × bohemica reduce the germination and growth of native plants. (2) Native plants germinate and grow less well on soils with a history of Fallopia × bohemica.
Materials and methods Plant materials and soil treatmentsFallopia × bohemica (Bohemian knotweed) is a hybrid between Fallopia japonica (Japanese knotweed) and Fallopia sachalinensis (Giant knotweed), two tall perennial Polygonaceae which were introduced to Europe from Eastern Asia at the beginning of 19th century as ornamentals. Although both parental species are well-known for their vigorous growth and clonal spread, the hybrid appears to even surpass the vigour and rate of spread of its parents (Mandak et al. 2004), and it is expected to eventually become the most abundant and problematic of the invasive knotweeds.
We used plant material from an invasive population of Fallopia × bohemica (hereafter Fallopia)located along the river Birs, close to Delémont, Switzerland (47°22.29'N, 7°21.26'E). This population has already served as a source of plant material for previous studies (e.g. Murrell et al. 2011), and its hybrid identity has been verified through molecular methods (
To create a litter leachate of Fallopia litter we followed the methods of
To test for possible allelopathic effects of persistent soil contaminants of Fallopia, we used the soil training approach (e.g.,
We selected nine native plant species as targets: two grasses (Lolium perenne, Poa trivialis) and seven forbs (Filipendula ulmaria, Geranium robertianum, Geum urbanum, Glechoma hederacea, Silene dioica, Symphytum officinale and Urtica dioica). All of these species commonly occur in habitats invaded by knotweed (
To investigate potential allelopathic effects of litter leachates and trained soil on the germination of the target species, we carried out a germination experiment in a greenhouse. In June 2009 we sowed seeds of each of the nine native species in 1-L pots filled with three different substrates: (1) a 1:1 mixture of sand and field soil, (2) the same mixture, but with litter leachate added twice (50 mL/pot each time), before and three days after sowing, and (3) the trained soil described above. To half of the pots in each substrate treatment we added activated carbon (Charcoal Activated, Merck KGA, Darmstadt, Germany) at a concentration of 20 mL/L. Activated carbon (AC) has a high capacity to adsorb organic compounds, and it can therefore be used to test for the presence of allelochemicals in the soil (e.g.
We sowed 20 seeds into each pot and covered them with a thin layer of identical soil, to avoid increased light absorption of the mixtures that contained AC and were thus slightly darker. There were 10 replicates per species × treatment combination, and thus a total of 540 pots (9 species × 3 substrates × 2 AC treatments × 10 replicates). The pots were arranged in a fully randomized order inside an unheated greenhouse under white shading cloth (60%). During the following eight weeks, we recorded germination every second day. In August 2009, when hardly any further germination could be observed, we thinned down each pot to the largest seedling and allowed this seedling to grow for another six weeks. After that, we harvested the aboveground biomass of the seedlings, dried them at 80°C for 72h, and weighed them.
Community experimentTo examine potential allelopathic effects of litter leachates and trained soil on the growth of established native communities, we carried out an additional mesocosm study in the garden. In June 2009, we planted artificial communities of five native forbs (Geranium robertianum, Geum urbanum, Glechoma hederacea, Silene dioica, Urtica dioica) into 4-L pots filled with the same 1:1 mixture of sand and field soil as above. In each pot, we planted five seedlings (one per species) in a circle, with randomized species order. There were four Fallopia treatments: (1) no Fallopia (= controls), (2) a piece (6–10 cm, two nodes) of Fallopia rhizome planted 5 cm deep in the centre of the pot, (3) 500 mL litter leachate added to each pot right after planting of the natives and the same amount two weeks later, and (4) the regular substrate replaced with trained soil as described above. To half of the pots in each treatment we added AC at a concentration of 20 mL/L. There were 11 replicates for each treatment by AC combination, a total of 88 pots. The pots were placed on root barrier (Plantex® Gold, DuPont, Wilmington, USA) in an experimental garden, with fully randomized order. The experiment lasted from June 2009 to June 2010. To avoid nutrient depletion, we fertilized all pots once in early 2010, using liquid fertilizer (N-P-K ratio: 7–5-6) equivalent to 25 kg N/ha.
During the spring of 2010, we recorded whether and when plants flowered. In June 2010, we cut the aboveground parts of all plants, dried them at 80°C for 72h and weighed them. In addition to flowering time and biomass, we recorded for each species the most feasible measure of reproduction: numbers of flowers for Geranium, Geum and Silene, number of flowering shoots for Glechoma, or numbers of inflorescences for Urtica. For Glechoma, the only species in our experiment that also reproduced vegetatively (stoloniferous spread), we also counted the numbers of runners, and calculated the ratio between runners and flowering shoots as a measure of allocation to vegetative versus sexual reproduction. Finally, we used the biomass data to calculate total community biomass as well as Shannon diversity (using species biomasses as abundances) for each pot.
Statistical analysesThe data from the germination experiment were analysed with linear models that tested the effects of soil treatments (control, litter leachate, trained soil), activated carbon, and their interactions. For germination rates, we used a generalized linear model (GLM) with quasibinomial error distribution, whereas the seedling biomass data were log-transformed and analysed with regular linear models. The two other types of germination data – time to first germination and germination half-time – were extremely ill-distributed and heteroscedastic and we therefore dropped them from the analyses.
The data from the community experiment were analysed with linear models that tested the effects of Fallopia treatments (control, rhizome planted, litter leachate, trained soil) activated carbon, and their interactions. First, we analysed total aboveground community biomass and community diversity. Second, we analysed the biomass and reproduction of each species individually. For reproduction, which was always count data, we used GLMs with quasipoisson error distribution, whereas biomass data were log-transformed and analysed with regular linear models. For most species in the community experiment, analyses of flowering time turned out to make little sense, because not enough plants flowered, or all flowered within a short period of time. The only species with a reasonably complete data set for analysis of flowering time was Silene, and we therefore restricted analyses of flowering time to this species. The flowering time data were analysed using GLMs with quasipoisson error. Finally, we analysed the clonal:sexual reproduction ratio of Glechoma using GLM with quasibinomial error distribution.
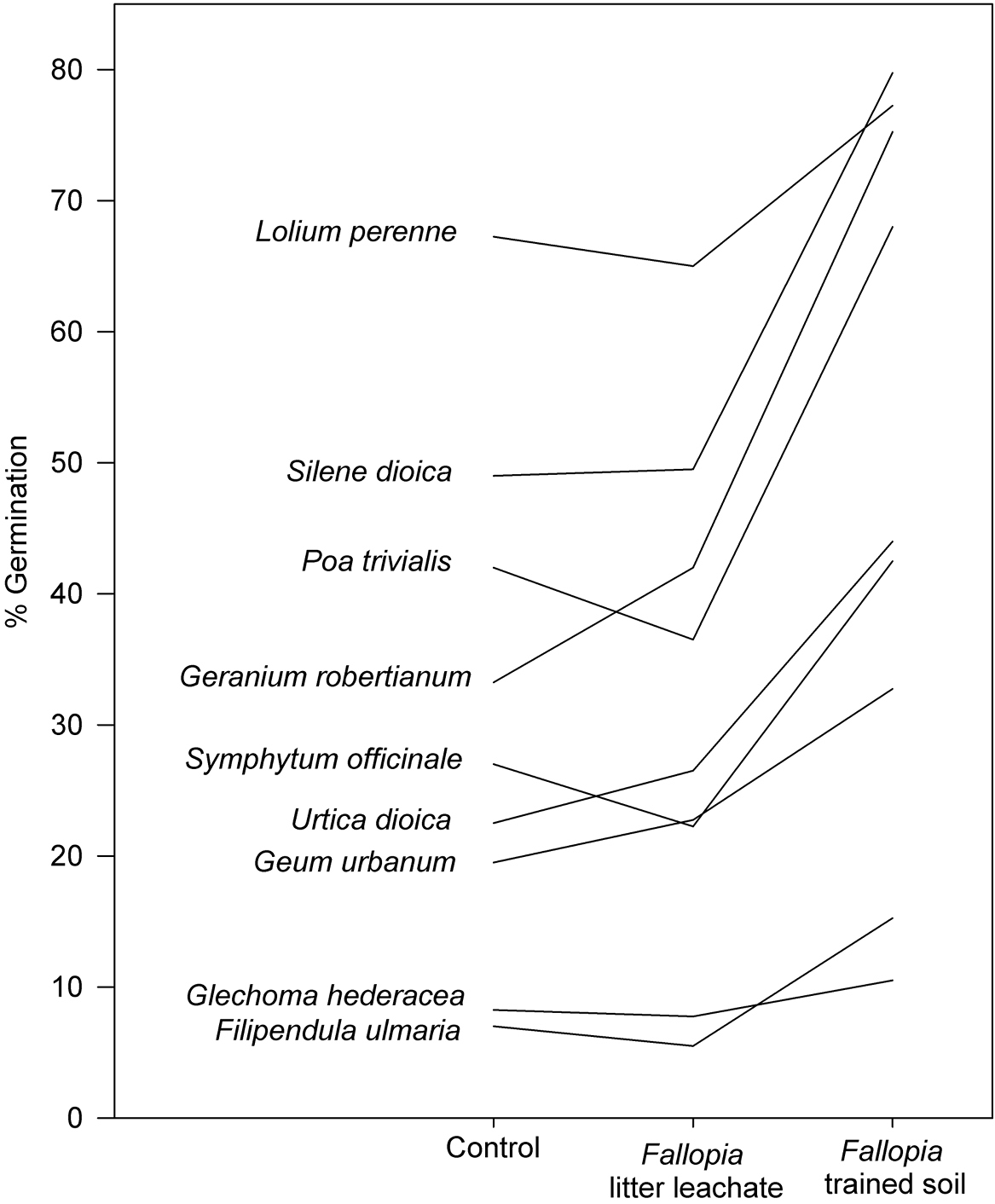
Results Germination experimentWe found significant effects of soil treatments on seed germination in seven of the nine target species (Table 1). However, in all cases these effects were due to positive effects of trained soil on seed germination (Fig. 1), whereas we never observed significant negative effects of litter leachates or trained soil on seed germination (all post-hoc tests non-significant for cases with a negative trend). There was a significant main effect of AC on Geum, where the percent of germinating seeds increased from 13 to 36. In two species, we observed a significant treatment by AC interaction. In the control treatment, the addition of activated carbon increased germination of Silene from 40% to 60%, but in the other two treatments, it did not have any effects. The interaction was more complex in Glechoma, where AC increased germination in the control treatment (5% to 11%) but decreased it in the litter leachate (11% to 5%) and trained soil (14% to 7%) treatments. There were generally much fewer effects of soil treatments on seedling biomass. Only in Glechoma and Silene we found a significant treatment effect. In Glechoma, litter leachate and trained soil increased seedling biomass by 27% and 100%, respectively, whereas in Silene the same treatments decreased seedling biomass by 22% and 27%. There were no effects of AC, or its interaction with the soil treatments, on seedling biomass in any of the species.
Analyses of variance of the effects of soil treatments (control, Fallopia × bohemica litter leachates, Fallopia × bohemica trained soil), activated carbon (AC), and their interaction, on the germination and early growth of nine native European species. *** P<0.001, ** P<0.01, * P<0.05. d.f. = degrees of freedom.
| Germination rate | Seedling biomass | |||||
|---|---|---|---|---|---|---|
| Species |
Treatment (d.f. = 2) |
AC (d.f. = 1) |
Treatment × AC (d.f. = 2) |
Treatment (d.f. = 2) |
AC (d.f. = 1) |
Treatment × AC (d.f. = 2) |
| Filipendula ulmaria | ns | ns | ns | ns | ns | ns |
| Geranium robertianum | *** | ns | ns | ns | ns | ns |
| Geum urbanum | * | ** | ns | ns | ns | ns |
| Glechoma hederacea | ns | ns | * | *** | ns | ns |
| Lolium perenne | *** | ns | ns | ns | ns | ns |
| Poa trivialis | *** | ns | ns | ns | ns | ns |
| Silene dioica | *** | ns | ** | ** | ns | ns |
| Symphytum officinale | *** | ns | ns | ns | ns | ns |
| Urtica dioica | *** | ns | ns | ns | ns | ns |
The effects of litter leachates and trained soil of Fallopia × bohemica on the germination rates of nine native European species.
In all of our experimental communities, Silene became the dominant species (average of 46.2% of the biomass across all treatments), followed by Glechoma (32.4%), Urtica (8.1%), Geum (5.6%) and Geranium (2.3%). This ranking was very stable and hardly affected by the treatments. In all of the 22 pots where we had planted Fallopia rhizomes, Fallopia resprouted, and it eventually constituted an average of 6.76% of the final community biomass. Overwinter survival exceeded 90% for all native species and did not differ across the four treatments. All individuals of Silene flowered in 2010, whereas flowering rates were lower in the other species (Glechoma 77%, Geranium 50%, Urtica 40%, Geum 30%). Out of the 22 planted Fallopia plants, none flowered until June 2010.
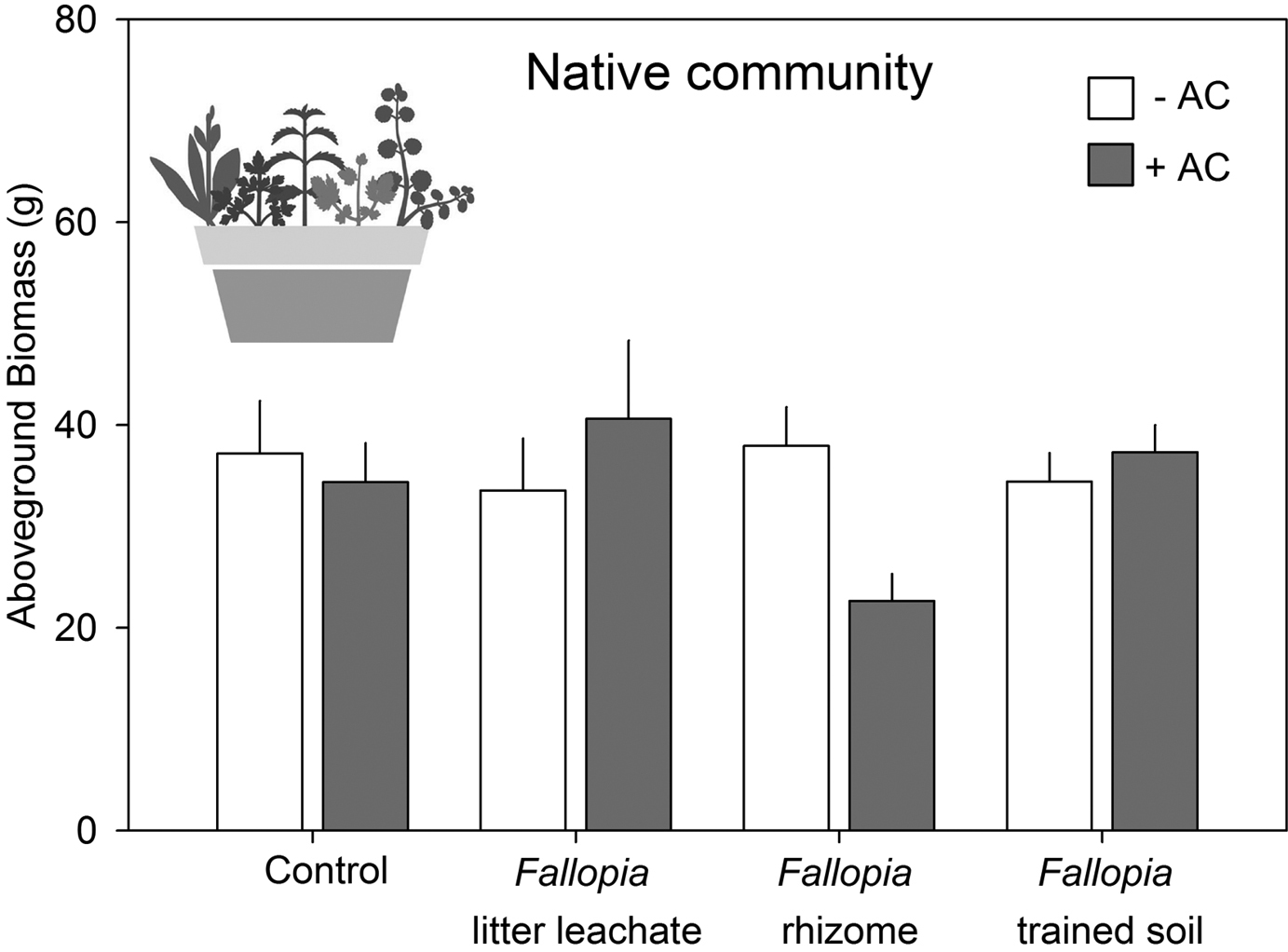
We did not find a significant main effect of the Fallopia treatments or AC on total native biomass (Table 2). However, there was significant interaction between Fallopia treatments and AC (Table 2, Fig. 2): while the addition of AC did not affect community biomass in the control or trained soil treatments, it strongly decreased (-45%) community biomass where Fallopia rhizomes had been planted, and it tended to increase (+14%) community biomass in the litter leachate treatment. There was a marginally significant (P = 0.065) effect of Fallopia treatments on the (biomass-based) Shannon diversity of the native communities, which decreased from 0.8 in the controls to 0.73 and 0.71 in the litter leachate and rhizome treatments, respectively, but increased to 0.92 in the trained soil treatment.
Native plants community biomass and diversity as well as individual species biomass in response to Fallopia × bohemica litter leachate and trained soil, with or without activated carbon (AC) added to the soil. Main effect and their interaction tested by factorial ANOVA. The values are F-values. ** P<0.01, * P<0.05, (*) P<0.1. d.f. = degrees of freedom.
|
Treatment (d.f. = 3) |
AC (d.f. = 1) |
Treatment × AC (d.f. = 3) |
|
|---|---|---|---|
| Total native biomass | 1.24 | 0.79 | 3.29* |
| Shannon index | 2.5(*) | 0.01 | 1.96 |
| Silene dioica | 0.19 | 0.84 | 0.58 |
| Urtica dioica | 2.16 | 1.13 | 2.47(*) |
| Geranium robertianum | 1.90 | 1.64 | 2.05 |
| Glechoma hederacea | 3.03* | 10.66** | 1.98 |
| Geum urbanum | 0.33 | 0.67 | 0.27 |
The effects of different possible sources of allelopathy of Fallopia × bohemica on the total aboveground biomass of a community of five native European species with or without activated carbon (AC) added to the substrate.
When we analysed the biomass responses of the native species separately, we found that the Fallopia treatments significantly affected the biomass of Glechoma, which, compared to the controls, had 43% less biomass in the rhizome treatment, but no significant change in the other two treatments. We also found that Glechoma biomass was consistently decreased by the addition of activated carbon (average of -33% across treatments). There were no other significant treatment or AC effects on the biomass of any other native species (Table 2).
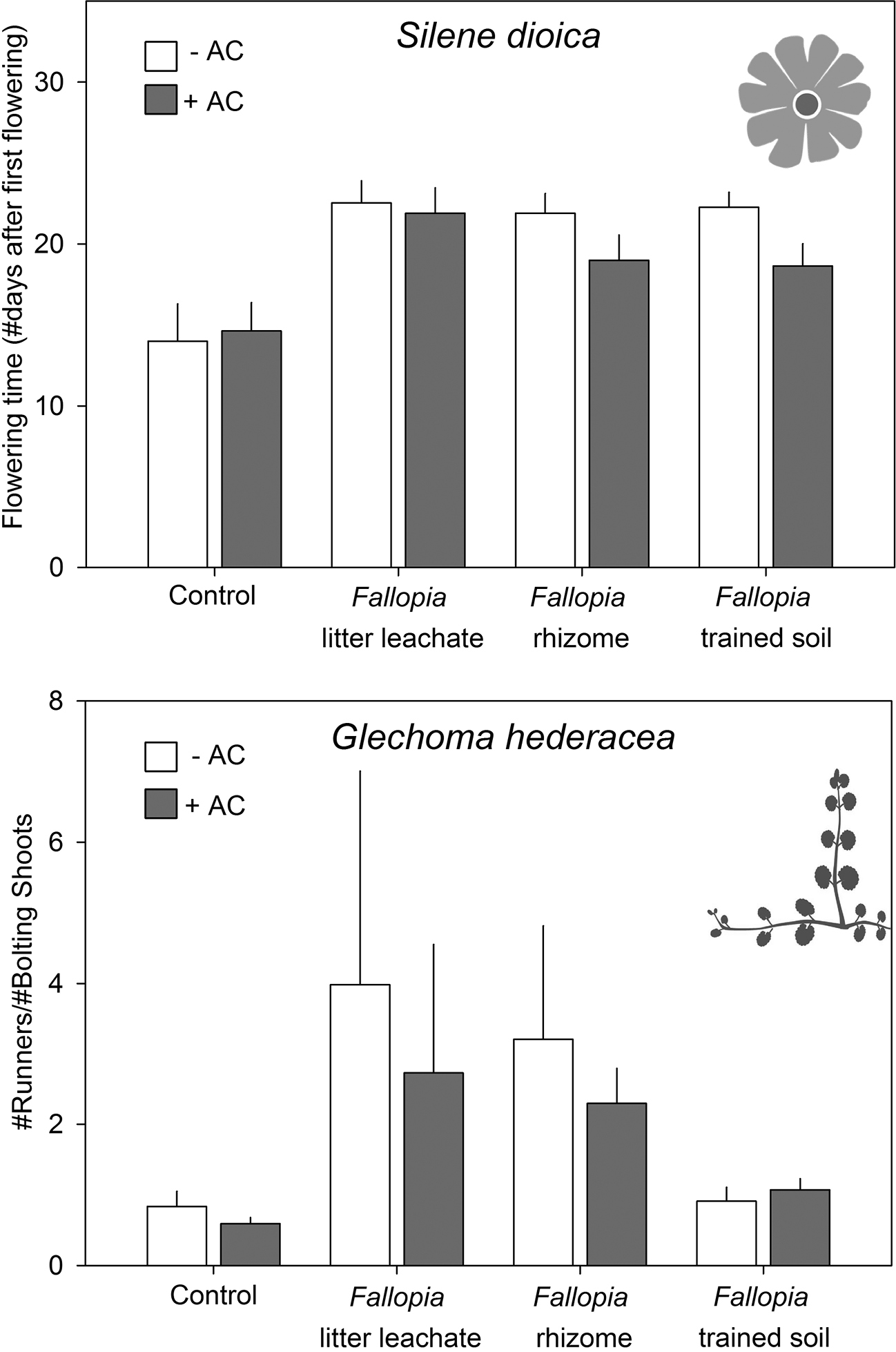
We also found significant treatment effects on reproductive traits for several of the natives. In all allelopathy treatments, the start of flowering of Silene was significantly (F = 9.92, P<0.001) delayed, and this effect was ameliorated by AC in the rhizome and trained soil treatments (Fig. 3).Moreover, addition of AC significantly increased the numbers of Silene flowers across treatments (F = 4.43, P<0.05). There was also a significant (F=3.69, P<0.05) treatment effect on reproduction in Glechoma, where the number of flowering shoots strongly decreased (from 17 to 6) where Fallopia rhizomes were planted. The number of runners, however, was not equally affected, which resulted in a significant (F = 4.69, P<0.05) shift of the ratio Glechoma runners: flowering shoots. With Fallopia rhizomes and litter leachates, this ratio was greatly increased (Fig. 3). Last, we found a significant (F = 4.02, P<0.05) treatment by AC interaction for Urtica flower biomass: addition of AC increased reproduction in the control and rhizome treatments, but decreased it in the two other treatments.
The effects of different possible sources of allelopathy of Fallopia × bohemica on the flowering phenology of Silene dioica, and allocation to vegetative reproduction of Glechoma hederacea, with or without activated carbon (AC) added to the soil.
Understanding the mechanisms of interference between successful invasive plants and their native competitors is key to explaining and ultimately managing plant invaders. Here, we experimentally examined whether one of the world’s worst plant invaders, the invasive knotweed Fallopia × bohemica, can exert allelopathic effects on natives also through its leaf litter or trained soil. We found little effects on the germination or biomass of natives, but both Fallopia litter extracts and trained soil caused significant life-history shifts in the dominant native species.
Germination experimentExposing native species seeds to Fallopia litter leachate and trained soil did not have any negative impact on their germination or early growth. In fact, trained soil even significantly increased germination rates of most native species. It is possible that the pre-cultivation of soil with Fallopia generally stimulated the soil microbial community, with positive consequences for seed germination, either because seeds have a greater chance of encountering the mutualists required for germination, or because more abundant soil microbes improve the water conditions in upper soil layers (
By setting up artificial native communities, we were able to evaluate the allelopathic potential of Fallopia in an ecologically meaningful set-up. In the community experiment, we found that neither planted Fallopia rhizomes, nor litter leachates or trained soil had a negative effect on the total biomass or diversity of the native community. Moreover, in none of the Fallopia treatments did addition of AC lead to increased native plant biomass or diversity, which would have indicated allelopathic effects. On the contrary, in the presence of Fallopia rhizomes, addition of AC even significantly decreased native plant biomass, which indicates beneficial chemical interactions between soil organisms and the native community, or between different native plant species, which were disrupted by AC. Taken together, our study provides no evidence for allelopathic effects of Fallopia at the level of the whole native community.
In our study, even the planted Fallopia rhizomes did not have allelopathic effects on native plants. This result is inconsistent with a previous study in which we found strong allelopathic effects of planted Fallopia (using the same knotweed genotype) on native community biomass (
We should stress that the first argument, lack of impact because of small size, applies only to the rhizome treatment, but not to the litter leachate and soil training. For the litter leachates, we followed the successful methods of previous studies (
Even though the total biomass of the native community was unaffected by the allelopathy treatments, such stability at the community level could mask underlying responses at the level of individual species. When we analysed the biomass responses of each species separately, the only species that showed a significant response to the experimental treatments was Glechoma, which had reduced biomass in the presence of Fallopia rhizomes or litter leachate. The reduction of biomass was stronger in the presence of AC, which suggests that AC may in fact have neutralized allelopathic compounds of Glechoma (rather than Fallopia) and thus reduced its competitive ability. Since Glechoma is one of the dominant species in the community, and the patterns of biomass change of Glechoma were similar to those of the whole community, it appears that biomass responses at community-level were largely driven by the responses of Glechoma.
In two of the natives, the dominant species Silene dioica and Glechoma hederacea, we looked also beyond biomass and reproduction and investigated allelopathic effects on key life-history traits, and we found that these were indeed strongly affected by the Fallopia treatments. In Glechoma, the only stoloniferous species in our experiment, with a clear dimorphism between shoots that become (vertical) flowering shoots and such that become (horizontal) runners, shoot allocation to runners was strongly increased both in the presence of Fallopia rhizomes and litter leachate. Such increased investment into runners with fast lateral growth can be interpreted as a switch towards a guerrilla strategy of growth (
In both cases where we found these shifts in life-history strategy, addition of AC tended to counteract these effects, which suggests that in both cases treatment effects must indeed be chemically mediated. Since the treatments generally did not affect plant biomass, the observed changes in allocation or phenology are not just allometric consequences of changes in plant size. For the chemical mechanisms behind these effects, there are several potential candidate classes of substances, including stilbenes, resveratrolosides and proanthocyanidins, which have been found in Fallopia and which were previously shown to be allelopathic in bioassays (Fan et al. 2010). As many of these compounds have antimicrobial and antifungal properties (
Our experiments show that Fallopia allelopathy acts on the growth of natives rather than their germination. Persistent soil contaminants appear to have rather limited effects on later life-history stages and this should not increase the efforts of restoring habitats after removing the invader. We also demonstrated that allelopathic effects can sometimes be subtle changes in life-history traits, which would be overlooked by a simple focus on plant biomass.
This research was supported by Swiss National Science Foundation grant 31003A_122408. We would like to thank one anonymous reviewer for the helpful comments.