(C) 2012 Álvaro Alonso. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
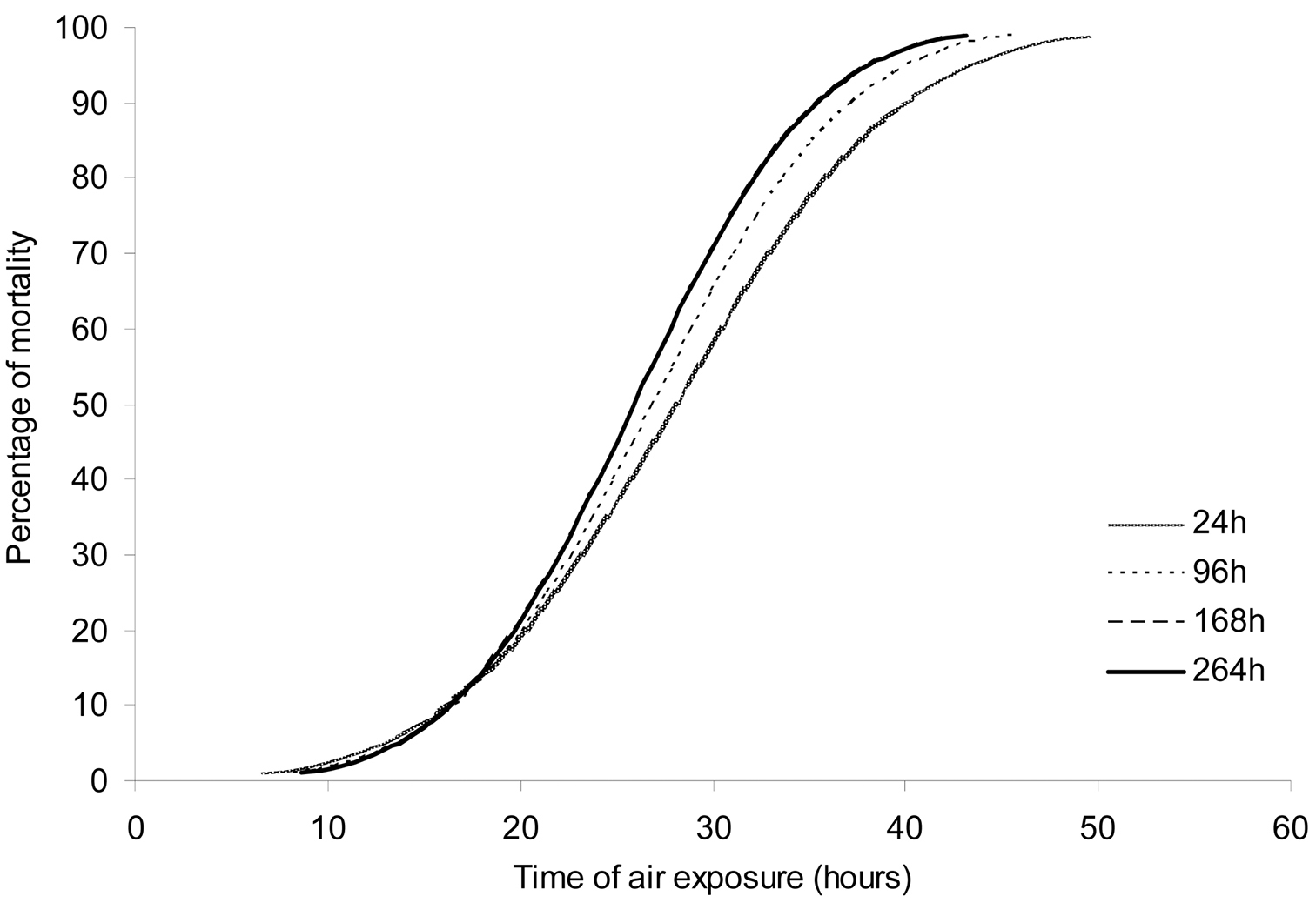
Spreading throughout a new ecosystem is the last step of an exotic species to become invasive. In the case of invasive aquatic molluscs, tolerance to air exposure is one of the main mechanisms allowing overland translocation and spreading. The mudsnail Potamopyrgus antipodarum (Hydrobiidae, Mollusca) is native to New Zealand but it has spread worldwide, invading ecosystems in Europe, Australia, America and Asia. The aim of our study is to assess mudsnail tolerance to air exposure, which may contribute to the successful overland translocation of this species. We conducted a laboratory experiment with four levels of air exposure (9, 18, 24 and 36 hours in a controlled climatic chamber). Snails were placed for 60 seconds in a laboratory paper filter to remove surface snail water. Then they were placed back in empty vessels during the four periods of air exposure, except the control group, which was immediately returned to water. At the end of each period of air exposure all vessels were filled with water and the cumulative mortality was monitored after 24, 96, 168 and 264 hours of rehydration. The calculated Lethal Times (i. e. the time of air exposure (in hours) necessary to cause the death of 50% (LT50) or 99% (LT99) of the population) and their 95% confidence limits at 24, 96, 168 and 264 hours were 28.1 (25.2–31.9), 26.9 (24.2–30.1), 25.9 (23.4–28.9) and 25.9 (23.4–28.9) hours, respectively for LT50, and 49.6 (42.7–63.3), 45.6 (39.9–56.5), 43.2 (38.0–53.0) and 43.2 (38.0–53.0) hours, respectively for LT99. Therefore an air exposure time over 43 hours caused the death of all studied individuals during all monitoring periods. Extending the monitoring period beyond 24 hours did not significantly change lethal times. Therefore, we recommend exposing fishing tools or boats at open air during at least 53 hours as a low cost measure to control mudsnail spread in early stages of invasion.
Exotic species, aquatic ecosystem, mudsnail, desiccation, spread
Biological invasions are one of the most important ecological problems at worldwide scale (
A rapid spread is the previous step to consider an exotic species as invasive. In the case of aquatic molluscs, tolerance to air exposure (i.e., absence of contact with water during a period of time) is one of the main prerequisites allowing overland transport, because many vectors require the ability to survive out of water (e.g., attached to nets, boats, buoys, waterfowl, etc.) (
The mudsnail Potamopyrgus antipodarum (Hydrobiidae, Mollusca) is native to New Zealand but has spread worldwide, invading ecosystems in Europe, Australia, America and Asia (
Previous studies on aquatic invasive molluscs have shown contrasting tolerances to air exposure among different species and environmental conditions. In the case of the mudsnail there are two studies for American populations (
The snails used in the experiment (mean length size of 3.8 mm, total number of 120) were obtained from our laboratory culture at the Department of Ecology (University of Alcalá). The culture was initiated with snails collected from the natural unpolluted upper reach of the Henares River (Guadalajara, Spain) in January 2009. Snails were kept in 60L glass aquaria with USEPA moderately hard water (96 mg NaHCO3, 60 mg CaSO4*2H2O, 4 mg KCl, 122.2 mg MgSO4*7H2O per litre of deionised water) (
Snails for the experiment were obtained from the culture and acclimatized to the experimental conditions (15°C air temperature) in a controlled climatic chamber (Ansonic VSC3207). Experimental water was the same as that in the culture aquaria. Snails were acclimatized over seven days before the experiment. During this period snails were normally fed. This temperature was selected as the average for spring conditions, as higher temperatures decrease their survival (
An air exposure experiment was conducted, using four different periods (0, 9, 18, 24 and 36 hours without water in a controlled climatic chamber), each in triplicate. Eight adult snails were used in each replicate (120 individuals altogether), which consisted of a glass vessel of 8 cm diameter and 6 cm height. Snails of each replicate and air exposure treatment were taken with forceps and placed for 60 seconds on a laboratory paper filter (Anoia Filter, 73g/m2), rolling them over the paper until no surface water was visible on their surface (i.e., snail shell without shine). After this preliminary dehydration, snails were placed in empty vessels for 9, 18, 24 or 36 hours, depending on the assigned treatment. Three additional replicates (with 8 snails each) were returned to the water as control treatment (0 hours of air exposure with preliminary dehydration). At the end of each air exposure period, vessels from each treatment were filled with control water (US-EPA water) and the cumulative mortality was monitored after 24, 96, 168 and 264 hours of hydration, including control treatment. Mortality was assessed by observing each snail under a binocular. An animal with a closed operculum was considered immobile and alive if after touching the operculum with forceps the snail retracted its soft body. If not it was considered dead. During the experiment air temperature and humidity in the climatic chamber were measured using a digital thermometer-hydrometer.
The lethal time 50 (LT50) and 99 (LT99) (i. e., the time of air exposure in hours that caused the death of 50% and 99% of the studied population, respectively) after 24, 96, 168 and 264 hours since the end of air exposure, and their respective 95% confidence limits were calculated using probit regression analysis (
The mean±SD (n=10) environmental conditions during the experiments were 68.9±6.6 % of relative air humidity and 15.4±0.5 °C of air temperature. The mortality of Potamopyrgus antipodarum was relatively low up to 20 hours of air exposure. After this time, mortality increased (Fig. 1). Air exposure times between 25.9 and 28.1 (LT50), and between 43.2 and 49.6 hours (LT99) caused the death of the half and all studied population, respectively (Table 1). An increase of the monitoring time neither changed LT50 nor LT99 values, as the confidence limits between different monitoring times overlapped (Confidence interval overlap test; p>0.05). Therefore, an air exposure treatment of 53 hours (upper limit of LT99 at 264 hours) assured the death of all snails.
Modelled probit curves for the percentage of mortality of Potamopyrgus antipodarum for each time of monitoring after returning the animals to water. Curves at 168 hours and 264 hours fully overlaped.
LT50 and LT99 values (in hours) at 24, 96, 168 and 264 hours for each time of mortality monitoring. 95% confidence limits are presented in parentheses.
| 24 h | 96 h | 168 h | 264 h | |
|---|---|---|---|---|
| LT50 (in hours) | 28.1 (25.2–31.9) |
26.9 (24.2–30.1) |
25.9 (23.4–28.9) |
25.9 (23.4–28.9) |
| LT99 (in hours) | 49.6 (42.7–63.3) |
45.6 (39.9–56.5) |
43.2 (38.0–53.0) |
43.2 (38.0–53.0) |
Our study demonstrated that survival of air-exposed mudsnail over short-period transport (<24 h) through non-aquatic media is highly probable. This scenario could be accomplished in waterfowl movements, transport of fishing tools (rubber boots, boats, nets, etc.) or movement of terrestrial animals (sheep, cows, domestic dogs, deer, etc.). Therefore the desiccation tolerance may be one of the most important traits promoting the traslocation of mudsnail in inland waters. In an American population
Air exposure caused several harmful effects in aquatic animals. In the case of mollusc a rapid loss of body water has been observed, being faster in small individuals than in large individuals (
The hard operculum of New Zealand mudsnail can contribute to explain its high tolerance to air exposure. In a previous study,
Avoiding the spread of invasive species is crucial for the conservation of aquatic native biodiversity. The results of our study suggest several low-cost measures to decrease the spread of mudsnail among reaches and aquatic ecosystems. First we recommend a desiccation treatment to all tools or instruments that are going to be repeatedly used in different aquatic ecosystems (e.g., exposing fishing tools at air during at least 50 hours, preferably at full sun light). Second, we recommend avoiding the access of wild and domestic animals to infected reaches or lakes (e.g., by using physical barriers or scarecrows for waterfowl). These simple measures may reduce mudsnail translocations in early stages of invasion.
This research was funded by the Spanish Ministry of Science and Innovation (SMSI) (CGL2010-16388/BOS) and by the Castilla-La Mancha Community (POII10-0179-470). The University of Alcalá provided logistical support. Dr. Álvaro Alonso was supported by a Juan de la Cierva contract from the SMSI at University of Alcalá. Many thanks to Enrique González for his help during the experiment. Especial thanks to Dr. Julio A. Camargo for providing climatic chamber for experiment.