A review of the influence of root-associating fungi and root exudates on the success of invasive plants
Introduction
Approximately 10% of non-indigenous plant species can significantly suppress or eliminate native populations (henceforth ‘invaders’), and have successfully established in regions around the globe, while others merely become naturalized and integrate with the native community (Richardson et al. 2000a). Explaining why some species are widely successful, with large impacts on native biota, is an active area of research (Rejmánek and Richardson 1996, Mack et al. 2000, Inderjit et al. 2008). In particular, herbaceous species such as Vincetoxicum rossicum, Euphorbia esula, Cirsium arvense, Alliaria petiolata, Polygonum cuspidatum, and Phragmites australis (dog-strangling vine, leafy spurge, Canada thistle, garlic mustard, Japanese knotweed, and common reed, respectively) have established widespread monocultures in Canada and become ecologically and economically problematic, threatening both native biota and reducing agricultural production (Lorenz 1991, Meekins and McCarthy 1999, Ailstock et al. 2001, Klironomos 2002, Kruger-Mangold et al. 2002, Cappuccino 2004).
The hypotheses explaining invader success are multifaceted and continue to evolve (Mack et al. 2000, Inderjit 2005). Explanations of why certain invaders successfully establish in novel ranges generally include reduced negative interactions or key facilitative interactions that allow certain species to overcome biotic barriers to invasion. A number of these theories attempt to explain invader success as a function of fungal associations, both beneficial and detrimental, or as a function of invader root exudates, which have also been shown to cause significant shifts in the life-history strategy of native plants such as reproductive timing and characteristics (Parepa et al. 2012). The Enemy Release hypothesis proposes that invaders flourish because they are released from natural enemies that are either not found or have not successfully adapted to the invader in the novel environments, including fungal pathogens (Keane and Crawley 2002). The Novel Weapons hypothesis suggests that invaders possess chemical exudates that are harmful to previously unexposed native organisms in the novel environment, thus disrupting plant communities and abetting invader establishment (Callaway et al. 2008). The Diversity-stability hypothesis holds that more diverse ecosystems are likely to contain at least one or more species prone to thriving under conditions of environmental perturbation, and are therefore able to fill niches of competitors that falter under such conditions—thus increasing resistance to invasion (Tilman and Downing 1994). Consequently, dampening of diversity due to the success of invading species can also result in ‘invasional meltdown’, whereby the proliferation of one invader can increase the likelihood of successful establishment of subsequent invaders (Simberloff and Von Holle 1999). As well, the concept of a ‘unifying theory’ was put forward by Hallett (2006), suggesting that successful invasions result from a combination of dislocation from antagonistic relationships, the availability of generalist microbial mutualists in the novel environment, and allelopathy.
The benefits attributed to mutualisms such as mycorrhizal (root-fungal) associations have recently become well represented in the literature (Johnson et al. 1997, Bever 1999, Smith and Read 1997). Similarly the advantages derived from powerful allelopathic and anti-pathogenic root exudates (novel weapons) have been linked to successful plant invasions. These effects are generally categorized as either i) benefits derived from associations with arbuscular mycorrhizal fungi (AMF) which are symbionts in the order Glomales, known to enhance nutrient and moisture uptake as well as provide protection from pathogens, or ii) benefits derived from root exudates that contain antibiotic (allelopathic, antipathogenic or anti-feedant) or signaling compounds. These investigations have typically been carried out in isolation of one another, although it is evident that there are distinct interdependencies and interactions operating between mutualistic and chemical strategies (Newsham et al. 1994, Klironomos 2002, Hallett 2006). Invaders are also suspected of being fungal generalists, a trait that can result in successful establishment and significant seedling recruitment, leading to the formation of monocultures and loss of community diversity.
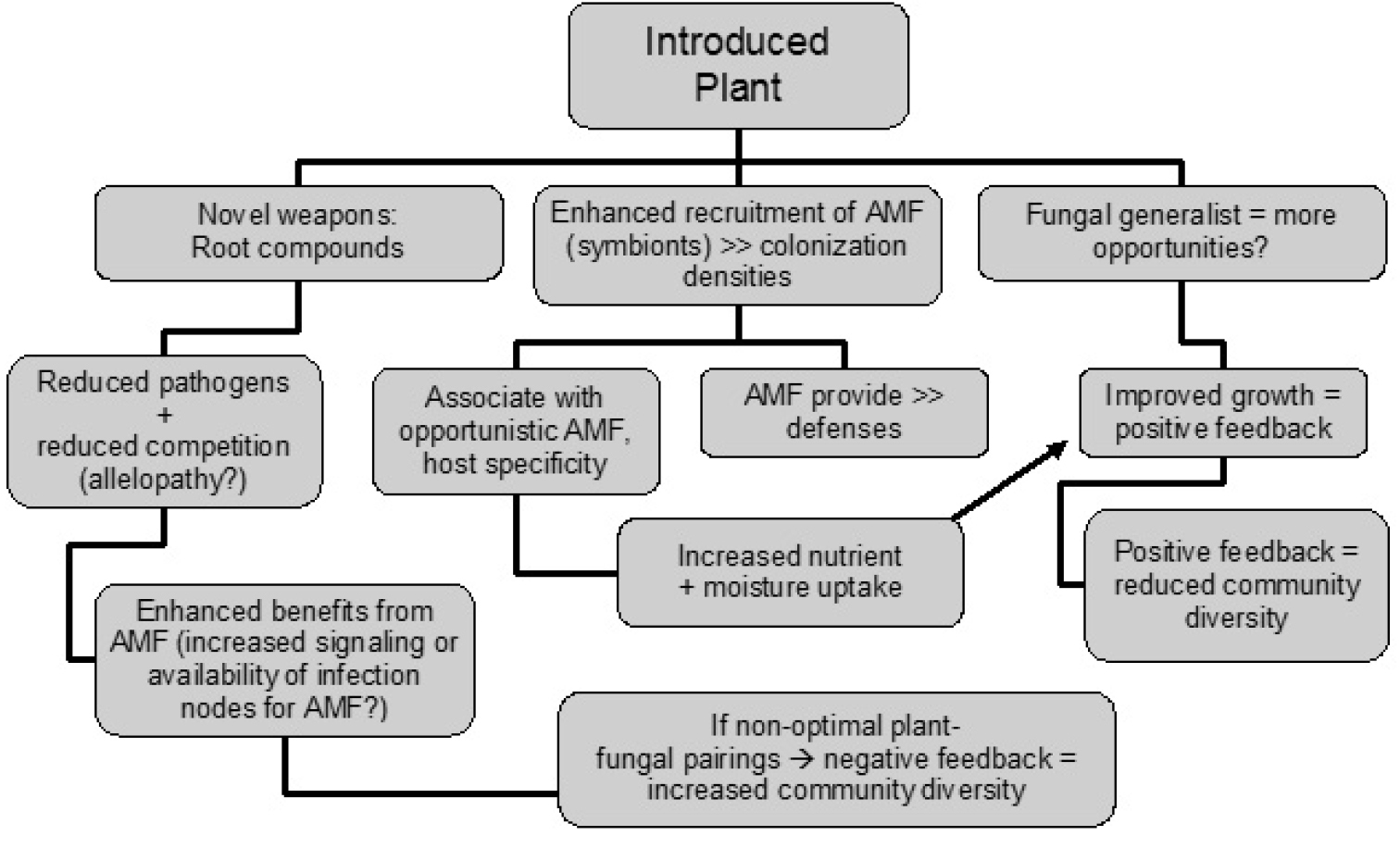
My objective in this review is to summarize the current understanding of plant-fungal associations in conjunction with root phytochemistry dynamics of successful invaders, and to suggest where research needs to be expanded in order to demonstrate the potential interdependencies between these two areas of research (see Figure 1). Knowledge of how they jointly influence invaders will further our understanding of processes supporting highly successful plant invasions.
Figure 1.
Possible dynamics within the rhizosphere and local soil environments subsequent to invasion by introduced plant species. The invader may possess novel or unique root compounds, an enhanced ability to establish associations with AMF, and/or the ability to associate with a wide range of beneficial fungi (some opportunistic P providers) relative to native plants.
AMF and invaders
Research related to plant-fungal association influences on invaders tends to bifurcate into two groups: investigations on fungi providing beneficial mutualisms, such as AMF and ectomycorrhizal fungi, and investigations on the impacts of parasitic/pathogenic fungi. In the case of the former, hyphal networks (vegetative filamentous structures) of mycorrhizae can extend as much as 200 times farther into the soil than the roots they colonize (Quinn 2011), providing increased resource availability that the plant would not otherwise have access to. Fungal partners may also mediate plant-to-plant transfer of carbohydrates and other nutrients among different individuals of the same plant species, as well as among different plant species within the community by linking together the roots of two or more plants. Such mycorrhizal communities are referred to as “common mycorrhizal networks” (Selosse et al. 2006, Egerton-Warburton et al. 2007). These generalist networks can result in the creation of inoculum reservoirs that promote selective seedling establishment within a plant community. However, plants differ in their responses to mycorrhizal fungi, and the fungi differ in their ability to distribute nutrients between coexisting plants. Arbuscular mycorrhizal plant-fungal symbioses form highly specialized nutrient-exchange structures (vesicles and arbuscules) at the plant-fungus interface and are recognized as being a particularly beneficial group of root endophytes. Plants associating with AMF are typically more competitive and better able to tolerate environmental stresses than are non-mycorrhizal or poorly colonized plants (Biermann and Linderman 1983, Daniell et al. 2001, Bianciotto and Bonfante 2002, Vandenkoornhuyse et al. 2002, Bonfante 2003, Brundrett 2004, DeBellis and Widden 2006, Greipsson and DiTommaso 2006, Parniske 2008, Shah et al. 2010), and AMF associations may contribute to the diversity of plant communities.
Bever (2002) noted that there is evidence of negative feedback on abundance of a given plant species resulting from the asymmetries between plant and AM fungus: plant-fungal associations are not always established in such a way as to maximize the benefit realized by either partner. For example, in the case where Plant-1 grows best when partnered with AMF-1, AMF-1 may be more inclined to establish associations with Plant-2, and so on. This dynamic is thought to contribute to the successful coexistence of a variety of plant species functioning within species guilds, thus preventing dominance by one or a few species. In this scenario, negative feedback due to non-ideal plant-fungal pairings is a mechanism that promotes diversity in plant communities. An introduced plant may disrupt this type of system balance where it differentially promotes or disrupts fungi in the plant community, suggesting that AMF research must be coupled with investigations on established plant-fungal community pairings within a local environment. These as well as other findings would indicate that species identity is also relevant to invasion biology when considering plant-fungal mutualisms (Crawley et al. 1999).
Root structure is another factor for consideration with regard to plant-AMF associations. Seifert et al. (2009) noted that introduced plants generally had finer root architecture relative to their native European counterparts, a characteristic consistent with a tendency to correspondingly increase reproductive biomass in newly introduced species. These findings suggest a reduced colonization by AMF in introduced plant variants, as finer roots are more typical of species with lower mycorrhizal responsiveness (Baylis 1975, Hetrick et al. 1992, Vogelsang and Bever 2009). Given that the structure and development of mycorrhizal hyphae is substantially altered in the presence of roots of host plants (Brundrett 2009), a shift in plant species representation post-invasion has the ability to significantly impact local AMF.
Plant community structure can be influenced by the presence/absence, density, and species richness of AMF in local soils, as plants can differ in their response to AMF colonization (Klironomos 2003). Less competitive plant species may establish a foothold within a community where they otherwise may have failed to persist in the absence AMF in the rhizosphere, suggesting that AMF promote plant coexistence and therefore contribute to community diversity (Moora and Zobel 1996, van der Heijden et al. 1998). For example, when small heal-all Prunella vulgaris plants were inoculated with AM fungi, they were able to successfully compete with larger neighbouring woodland strawberry Fragaria vesca plants that they had failed to successfully compete with pre-inoculation in greenhouse experiments (Moora and Zobel 1996). AMF associations would therefore increase biodiversity by decreasing the interspecific suppression of small plants by larger neighbours. It should be noted that fine-scale studies look at the impact of composition and diversity of AMF rather than just presence or absence of AM fungi (Hart et al. 2003), and therefore these studies consider factors such as host specificity and have more relevance for later-successional stages of invader range expansion. It has been proposed that AMF may be either disrupted or harnessed by aggressive invasive plant species, resulting in altered native fungal communities in the soil as the invader attains dominance in the system (van der Heijden et al. 1998, Daniell et al. 2001, Griepsson and DiTommaso 2006, Bastias et al. 2007, Curlevski et al. 2010).
The mechanisms by which AMF association provides advantages to successful invaders as they establish and attain dominance in a novel range typically include: i) increased general AMF colonization density of invaders relative to natives can lead to improved nutrient and moisture uptake and increased competitiveness, ii) host specific AMF that support plant community structure and diversity can be significantly altered by invaders, and iii) pathogen protection by AMF may be provided selectively to invaders.
Key findings for each category are summarized below.
AMF colonization, dependence, and density
Some aggressive plant invaders have been observed to sustain significantly increased AMF colonization densities relative to local native plants in the field. An example of this is Vincetoxicum rossicum, colloquially known as dog-strangling vine (DSV) because its twining tendencies lead to dense interconnected mats of impenetrable vegetation. DSV has been shown to be more densely colonized by mycorrhizal fungi than co-occurring native plants in Henderson Harbour, New York (Greipsson and DiTommaso 2006, Smith et al. 2008). Smith et al. (2008) used bright field microscopy to detect evidence of significantly greater fungal colonization of DSV relative to leek Allium ampeloprasum bait plants (frequently used as a predictor of AMF density in soils), and local natives such as milkweed Asclepias syriaca, Canada goldenrod Solidago canadensis, or naturalized blueweed Echium vulgare L. Additionally, DSV colonized by fungal partners had a significantly greater total biomass relative to DSV grown in sterilized soil reamended with AMF-free microbial wash (Smith et al. 2008).
Soil microbial communities have been observed to change progressively as invasion advances from year to year (Wolfe and Klironomos 2005, Batten et al. 2006), and it has been suggested that invaders benefit disproportionately from symbiotic mutualists, particularly in disturbed sites (Reinhart and Callaway 2006, Sun and He 2010). Plants are capable of controlling the density of mycorrhiza representation by root growth, digestion of old interface hyphae in plant cells, or altered root system formation (Brundrett 2008). These types of mechanisms may be at play in the field when invasions occur, followed by shifts in fungal colonization patterns. A study by Liang et al. (2004) showed a significant positive correlation between invasion time and AMF colonization rate of goldenrod Solidago canadensis, a successful invader in China, though there was differential colonization evidenced by the different AMF species, depending on the environmental characteristics. They noted that one species of AMF, Glomus mosseae, demonstrated increased colonization density over time in Solidago canadensis, while another AMF species, Glomus constrictum, showed decreased colonization density over time. It was suggested that Glomus constrictum may be an early successional species, and Glomus mosseae a late-successional species. The authors concluded that AMF aided establishment and proliferation of Solidago canadensis in its introduced range in China.
In contrast to the above studies, soil/AMF conditioning studies and bioassays by Vogelsang and Bever (2009) demonstrated that the invasive herbaceous forb Italian thistle Carduus pycnocephalus exhibited strong growth in soil lacking arbuscular mycorrhizal fungi, as well as in soil conditioned by a diverse mix of non-native plant species. As well, Carduus pycnocephalus growth was inhibited by the soil that best promoted the native herbaceous forb California cudweed Gnaphalium californicum. Mycorrhizal density investigations showed a reduction of AMF in the invader-conditioned soil relative to the native-conditioned soil, suggesting that in some cases invader species do not promote the growth of mycorrhizal fungi in the same way that native species do. Given that most crop plants are hosts to AMF, this association is potentially an important resource for agriculture (Sieverding 1991, Oehl et al. 2003), and should be considered for further study.
AMF host specificity, richness, and opportunism
Given the observed low AM fungi:host plant ratio (i.e. approx. 150 described AMF species to 300, 000 plant species), it is generally assumed that there is a high functional redundancy among AM fungal species, and therefore low host specificity (Klironomos 2000). However, it has been shown that there are differential phosphorus uptake and pathogen protection responses that are highly plant- and fungus-species specific for different plant-fungus combinations (van der Heijden et al. 1998, Helgason et al. 2002, Duponnois et al. 2005, Gustafson and Casper 2006, Gogoi and Singh 2011). Optimal plant-AM fungi combinations are more likely to be established where higher AMF species diversity is present. As such, mycorrhizal fungal species richness has been shown to affect plant productivity, and observed shifts and loss of diversity in AMF groups post invasion could impact plant community structure (Maherali and Klironomos 2007). It has been observed that certain aggressive invading plant species can alter the existing native fungal communities in a densely invaded environment, thus disrupting native plant communities (Helgason et al. 2002, Kourtev et al. 2002, Mummey et al. 2005, Batten et al. 2006, Greipsson and DiTommaso 2006, Hawkes et al. 2006, Stinson et al. 2006, Meinhardt and Gehring 2012). Hawkes et al. (2006) described a dramatic shift in the composition of AMF communities in native North American grasses Nassella pulchra and Lupinus bicolor subsequent to invasion by introduced grasses Avena barbata and Bromus hordeaceus. There was a shift away from species originally colonizing natives toward those colonizing the invader, and fungal species richness in the natives was reduced as a result of the shift (Hawkes et al. 2006). Compositional changes in AM fungi pre- versus post-invasion supports host specificity as a factor, given that reduction in native plant species representation correspondingly reduced the associating fungi. The authors concluded that invading plants could influence the network of mycorrhizal fungi available to natives, thus providing a mechanism for successful establishment and subsequent invasion. Similarly, Mummey et al. (2005) used terminal restriction fragment length polymorphism (T-RFLP) and multivariate analyses to show that AMF communities associating with Dactylis glomerata, a common forage grass species naturalized in mid-western US, shifted to reflect the community composition associated with Centaurea maculosa, a noxious weed native to eastern Europe, post invasion. As well, Stinson et al. (2006) observed that the non-mycorrhizal exotic invasive forb garlic mustard Alliaria petiolata suppressed both AMF and ectomycorrhizal fungi, the latter being instrumental in tree seedling establishment and growth. Disruption of AMF by successful invaders could result in loss of natives dependent on such species, supporting competitive exclusion by the invader.
Vandenkoornhuyse et al. (2003) used PCA analyses to determine plant-fungal association patterns generated by T-RFLP using AMF-specific primers. They demonstrated that three different co-occurring grass species (Agrostis capillaries, Festuca rubra, and Poa pratensis) were differentially colonized by AMF phylotypes, suggesting that recognition mechanisms exist that confer fungal-host plant specificity. Another study by Helgason et al. (2002) similarly investigated host specificity in mycorrhizal fungal associations using small-subunit rRNA gene amplification and sequencing. They found that the only species of AMF colonizing field collected Acer pseudoplatanus in North Yorkshire (UK) was Glomus hoi, while several other species of AMF (Scutellospora dipurpurescens, Archaeospora trappei, and Glomus sp. isolate UY1225) were found differentially colonizing four neighbouring plant species (Rubus fruticosus agg. L., Epilobium angustifolium L., Ajuga reptans L. and Glechoma hederacea L.). The Glomus hoi consistently outperformed other AM fungi in improving P uptake in all the woodland plants in greenhouse experiments, thus enhancing the growth of these mycorrhizal plant species (Helgason et al. 2002), particularly in P-limited environments. Consequently, disruption or promotion of specific AMF by invaders would significantly impact existing plant community structure.
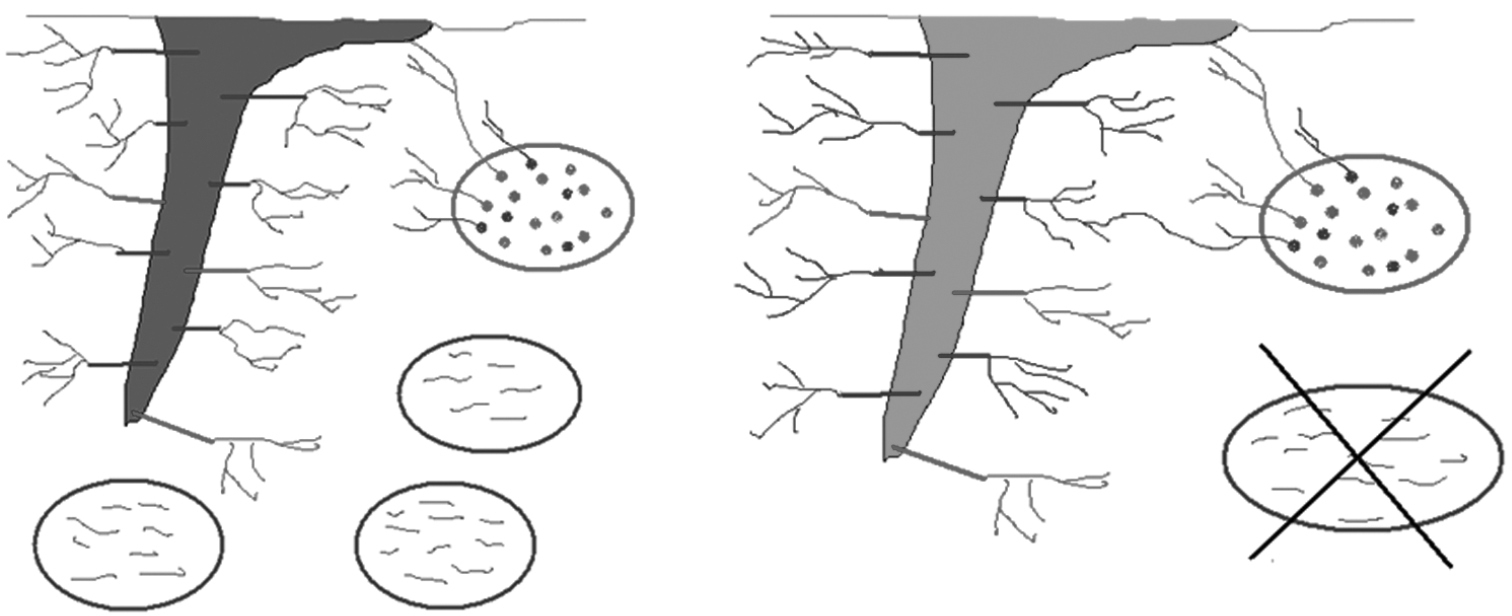
Greipsson and DiTommaso (2006) predicted that highly invasive species such as Vincetoxicum rossicum will tend to associate with fast-growing opportunistic or highly beneficial AMF species, enabling rapid establishment of invaders in their novel range. Glomus species have been shown to comprise a subset of such AMF because they have the advantage of readily forming anastomoses (connections between hyphae) with other fungi and are able to colonize via hyphal fragments, whereas other AM fungi typically require intact mycelia to colonize host plants, or direct infection from spore dispersal (Figure 2) (Biermann and Linderman 1983, Giovannetti et al. 1999, Fumanal et al. 2006). Glomus intraradices, Glomus caledonium, and Glomus mosseae were observed to form anastomoses between hyphae originating from the same spore as well as different spores from the same isolate, while this was not the case for Gigaspora or Scutellospora spp. tested (also AMF) (Giovannetti et al. 1999). As such, these Glomus species have been shown to behave opportunistically relative to commonly co-occurring AMF (Giovanetti et al. 1999, Helgason et al. 2002). As well, preferential P uptake by Glomus intraradices has been observed in several studies (Graham and Eissenstat 1994, Appoloni et al. 2008, Van Aarle et al. 2009), suggesting that this species of AMF would be particularly beneficial in low P environments. DSV was observed to associate with Glomus intraradices and Glomus caledonium in field studies in Southern Ontario, where several co-occurring native plants were not observed to form such associations based on molecular investigation of root-colonizing AMF communities (Bongard et al., unpublished data).
Figure 2.
The root on the left represents an invasive plant that is able to form associations with opportunistic AM fungal fragments, while the root on the right cannot form such associations, but must form associations with hyphae of germinating spores or intact mycelia (i.e. established hyphal networks). The ability to form these opportunistic associations with hyphal fragments would be highly advantageous in mechanically disrupted sites.
Glomus species with demonstrated ability to colonize roots via hyphal fragments also appeared to be more beneficial in promoting plant growth over Gigaspora species (Bever et al. 2009), which have no demonstrated ability to colonize roots via hyphal fragments (Biermann and Linderman 1983, Bever et al. 2009). Maherali and Klironomos (2007) also noted that members of the Glomaceae (includes Glomus species) have demonstrated reduced pathogen abundance in roots; however, Gigasporaceae generally enhanced nutrient uptake in their plant hosts. Highly beneficial influences on plant growth was observed in the shrub Acacia holosericea inoculated with Glomus intraradices in a study by Duponnois et al. (2005), who observed that Glomus intraradices optimized P solubilization and uptake from mineral phosphate. Maldonado-Mendoza et al. (2001) observed that a phosphate transporter gene (GiPT) in the extra-radical mycelium of Glomus intraradices was expressed during associations with carrot Medicago truncatula roots. Their analyses revealed that GiPT expression is regulated in response to phosphate concentrations in the environment and modulated by the overall phosphate status of the mycorrhiza, suggesting that Glomus intraradices can detect phosphate levels in the external environment, as well as having an internal phosphate sensing mechanism. This ability in the propagative units among glomalean families would provide enhanced early successional establishment opportunities for plants capable of forming associations with these species (Giovannetti et al. 1999). In heavily mechanically disrupted sites, mycorrhizal networks may be in a slow state of recovery and therefore respond in a manner similar to early successional behavior (Hart et al. 2003), suggesting that colonization via mycelial fragments, or opportunistic Glomus spp., may be the predominant method of forming associations. Where invaders are preferentially colonized by this subset of AMF, establishment advantages would be gained.
While plant-fungal host specificity has been described in various studies, it has been observed that some invaders are most likely fungal generalists (Richardson et al. 2000b, Miller 2008, Moora et al. 2011), a characteristic that may provide advantages for establishment and spread in a novel environment. For example, DSV has been observed to form associations with a greater variety of fungal colonizers relative to proximal native plants, and has also demonstrated greater fungal colonization densities (Smith et al. 2008, Bongard et al., unpublished data).
AMF pathogen protection
AMF species with the ability to protect their host plants from a variety of microbial pathogens have been described in the literature (Newsham et al. 1994, Borowicz 2001, Klironomos 2002, Pozo and Azćon-Aguilar 2007, van der Putten et al. 2007, Appoloni et al. 2008, Mogg et al. 2008, Krüger et al. 2009, Wehner et al. 2009). It has been suggested that plants associating with these highly beneficial AMF could have defensive enzyme and chemical production induced in their roots by their mycorrhizal partners, and subsequent damage to roots of non-host plants may occur while host roots remain protected (Brundrett 2004, Bais et al. 2006). Disruption of protective AMF by invaders would increase the susceptibility of natives that typically benefit from this type of protection, while the invader may simultaneously benefit from enemy release provided in the novel environment (Keane and Crawley 2002). Possible differential exploitation of these AM fungi by invaders would also provide advantages in the novel environment.
In a study using meta-analysis of AMF and plant pathogen data gathered from papers published between 1970 and 1998, it was determined that AMF tended to decrease the harmful effects of fungal pathogens (Borowicz 2001), and were shown to reduce parasitic nematode Meloidogyne incognita damage in white clover Trifolium repens L. (Habte et al. 1999). In experiments testing three AMF Glomus species(Glomus aggregatum, Glomus mosseae, and Glomus intraradices) for their relative effectiveness in providing protection against nematode infestation, Glomus intraradices was found to provide the most effective protection (Habte et al. 1999). The authors noted that colonization by Glomus intraradices in particular was enhanced in the presence of nematodes, and that nematodes did not have a significant influence on plants colonized by Glomus intraradices. Glomus intraradices has been described as a promiscuously associating generalist (Graham and Eissenstat 1994, Appoloni et al. 2008, Van Aarle et al. 2009).
Maherali and Klironomos (2007) found that increased Glomaceae richness in roots generally increased competition against pathogenic soil fungi in lab experiments. Shah et al. (2010) observed reduced Glomaceae representation in soils post invasion by two highly invasive European herbaceous species Anthemis cotula and Conyza canadensis (Asteraceae) using rhizosphere spore analysis, thus potentially resulting in the loss of AMF-induced pathogen protection to natives. As well, Vigo et al. (2000) observed that tomato plants Lycopersicon esculentum colonized by Glomus mosseae had reduced infection by the soil pathogen Phytophthora parasitica relative to plants not colonized by Glomus mosseae, with results showing 39% fewer infection loci from the pathogen seven days after zoospore inoculation with Glomus mosseae. These findings suggest that one mechanism by which AMF protect against fungal pathogen infection is via efficient colonization of the plant infection points or loci, which are limited in number. As such, this hypothesis supports the necessity of combining AMF colonization density studies with those investigating AMF host specificity and AMF as agents of protection. Wright and Upadhyaya (1998) hypothesized that glomalin, a glycoprotein unique to AMF, is used for pathogen protection of the AMF hyphae that extend into the soil and facilitate nutrient transfer. Sub-groups of AMF may also be activating defense mechanisms within the host plant: ‘defensin’, a cysteine-rich plant protein demonstrating anti-fungal activity was only upregulated in roots colonized by Glomus intraradices relative to roots that were colonized by Glomus mosseae (Wehner et al. 2009).
Though AMF have been shown to confer protection against pathogenic fungi, it has been observed that there could be synergistic and/or additive effects produced by different AMF species combinations (Gustafson and Casper 2006, Wehner et al. 2009). Wehner et al. (2009) hypothesized that protection against pathogens could be due to the combined effects of increased access to nutrients provided by AMF, enhancement of plant defense mechanisms stimulated by AMF associations, and altered root architecture (increased lignin concentrations) as a response to AMF colonization. However, depending on the soil environment and other factors, AMF can function on a continuum from parasitism to facultative exploitation by their plant hosts (Brundrett 2004); hence environment nutrient load must also be considered. Because of the variability in the benefits provided by AM fungi, it is important to understand more about the dynamics of the local fungal community as a whole, not just AM fungal density or dominant species type.
In planning studies on invasion success, it would be useful to consider multiple effects attributable to simultaneous colonization by AMF as well as other fungi, plant traits such as root architecture, and variation in benefits derived from different AMF species, which may or may not afford unique chemical protection to colonized roots (Seifert et al. 2009). Having a more complete picture of the plant-fungal dynamics would offer a better view to plant invasion processes, as well as elucidate how AM fungal specialists interact with the general (non-AMF exclusive) fungal community in supporting plant diversity and competition.
Fungal pathogens and invader root exudates
Fungal pathogens
Some invasive plant species have demonstrated significantly reduced infection by fungal pathogens in their introduced range relative to their native range, an effect that may not be directly attributed to associations with AMF (Keane and Crawley 2002, Callaway et al. 2003, Mitchell and Power 2003). This could be explained by the Enemy Release hypothesis described previously. However, Eppinga et al. (2006) used mathematical modeling to account for distribution patterns and success of the invasive species Ammophila arenaria (marram grass or European beach grass) in California that resulted from Ammophila arenaria’s ability to tolerate accumulation of local soil pathogens rather than enemy release. The authors hypothesized that while the accumulation of pathogens may initially limit invader abundance in the novel environment, it might feed back more negatively to the native plant community. As such, they concluded that in California Ammophila arenaria accumulated local generalist pathogens that had a more negative effect on the native plant species than on Ammophila arenaria. Alternatively, Blumenthal et al. (2009) examined the effects of fungal pathogens hosted by invaders in the US relative to the effects of the same plants in their native European ranges. Their results suggested that plants from resource-rich native environments (strong competitors) are more likely to experience release from pathogens in their non-native range than plants originating in low resource habitats (stress-tolerant plants). The authors hypothesized that successful invaders will possess high growth characteristics and demonstrate a lower pathogen burdens in their introduced range.
Root exudates
Plant root exudates are substances that alter the conditions of the rhizosphere by changing pH levels and mineral availability via desorption, as well as influencing the growth and interactions of microorganisms (Rovira 1969). Plant-plant interactions are often mediated by root exudates (Bais et al. 2006, Parepa et al. 2012). Allelopathy, the suppression of germination or growth of neighboring plants by the release of toxic secondary chemical compounds, has been explored as a pathway for successful plant invasion (Callaway and Aschehoug 2000, Inderjit 2005, Callaway and Howard 2007, Antunes et al. 2008, Douglass 2008, de Souza et al. 2010, Cantor et al. 2011). De Souza et al. (2010) used multiscale mathematical models to determine that plant invasion patterns and success based on allelopathy depends on both the nature of the local native plants in the invaded site, as well as the nature of the invader phytotoxins (root exudates). Both the celerity and success of invasion increase in the presence of weakly resistant and relatively homogeneous native plants and effective invader phytotoxins. For example, in the case of spotted knapweed Centaurea maculosa in the western US, invasion is particularly successful due to the phytotoxin (-)-catechin exuded from roots, which inhibits germination and triggers the death of root systems of susceptible native grass species (Bais et al. 2003). Allelopathy has also been suggested as a means of successful expansion of invaders in North America (Callaway et al. 1999, de Souza et al. 2010), including DSV (Milbrath 2010, Milbrath et al. 2011), as well as invaders in other countries such as Japanese knotweed Fallopia japonica in Wales (Hollingsworth and Bailey 2008) and Canada goldenrod Solidago canadensis in China (Zhang et al. 2009). Extracts of Solidago canadensis roots were also shown to significantly suppress the growth of soil oomycete and fungal pathogens Pythium ultimum and Rhizoctonia solani relative to extracts of common local native plants in culture experiments (Zhang et al. 2010).
Studies have shown that root exudates of invaders can decrease colonization by pathogenic fungi in their novel range (Bais et al. 2006, Mogg et al. 2008). However, the impacts of root pathogens can range from reduced plant growth and fecundity to total plant failure; the interaction of plant and pathogen depends upon the class of pathogen as well as the defense-relative-to-growth strategies of the plant. Strong competitors in resource rich environments tend to allocate resources to growth (large leaf area) rather than defense, but often cope relatively well with a high pathogen load, whereas stress-tolerant competitors that excel in resource poor environments tend to make use of defensive root exudates that keep pathogens in check (Seastedt 2009). Consequently, in resource rich novel environments with reduced plant-specific pathogens (release from enemies), invaders are able to become strong competitors, while in disturbed, resource poor novel environments, invaders can gain an advantage when their root compounds (anti-pathogenic or anti-feedant chemicals) are particularly effective against pathogens and herbivores.
Invader root exudates may disrupt AM fungal associations of nearby natives, thereby decreasing competitiveness (Vierheilig et al. 2003, Stinson et al. 2006), or even comprise chemicals that are both allelopathic toward neighbouring plants and disruptive to AMF (Zhang et al. 2010, Hale et al. 2011). Zhang et al. (2007) demonstrated that root extracts of Solidago canadensis added to several native plants in China (Echinochloa crusgalli, Kummerowia striata, and Ageratum conyzoides) significantly inhibited AMF colonization by several Glomus species common to natives one year post invasion. Broeckling et al. (2008) observed that two model plant species, Arabidopsis thaliana (non-mycorrhizal Brassicaceae) and Medicago truncatula (AMF-associating), demonstrated the ability to maintain associations with local soil fungus communities consistently over time, while not being similarly capable of maintaining non-resident soil fungus associations. The authors compared the resident soil fungus phylotype community profiles between native and non-native plant conditioned soils (or soils conditioned with their root exudates) using real-time PCR. The presence of non-native plants growing in soils, or their root exudates alone, influenced the fungal community by both positively and negatively impacting the relative abundance of individual phylotypes, while the native plants (or their root exudates) maintained consistent fungal community profiles over multiple plant generations. Accordingly, where an invader brings fungi new to the introduced range, natives dependent on resident fungal communities that are prone to disruption would be disadvantaged. As such, root exudates may serve as a selective agent enabling regulation of the fungal community in the rhizosphere (Broeckling et al. 2008). Roots of common milkweed Asclepias syriaca (in the same family as DSV, Asclepiadaceae) gained significant biomass when grown in DSV-conditioned soil relative to plants grown in uninvaded soils (DiTommaso 2006). This was attributed to a possible escape from fungal pathogens induced by the phenthorindolizadine alkaloid chemical (–)-antofine exuded by DSV roots (Mogg et al. 2008). DSV has demonstrated antifungal activity against plant pathogenic yeast-like and filamentous fungi, as well as broad-host-range plant pathogens such as Fusarium spp. (Mogg et al. 2008). These findings suggest that there is a strong likelihood that fungal communities associating certain aggressive invaders will differ from those associating with native plants that do not demonstrate similar phyto-chemistry, resulting in native community disturbance.
Stinson et al. (2006) reported that the mutualistic associations between native tree seedlings and mycorrhizal fungi were disrupted by the non-mycorrhizal European native garlic mustard Alliaria petiolata, resulting in decreased seedling survival. They found that hardwood seedlings grown in garlic mustard-conditioned soil showed significantly reduced AMF colonization of roots and slower growth than those grown in non-garlic mustard conditioned soil, based on lab experiments conducted in Waterloo, Ontario. The authors attributed the ability of Ammophila petiolata to dampen seedling growth of AMF-dependent competitors to the glucosinolate root exudates manufactured by these members of the Brassicaceae family. These findings were supported by Callaway et al. (2008), who found that the anti-fungal influence of Ammophila petiolata had far greater inhibitory effects in North America than in their native European ranges. Root exudates that are relatively harmless to resistant mycorrhizal symbionts in the home range as a result of adaptation, may therefore be disruptive to native mutualists in the introduced range, and indirectly suppress the plants that rely on them (Callaway et al. 2004).
Some plant root exudates have also been shown to increase the signaling pathways attracting AM fungi, and consequently increase hyphal growth and colonization by these beneficial symbionts (Vierheilig 2004, Akiyama et al. 2005, Greipsson and DiTommaso 2006). A net increase in fungal biomass was observed in the experiments by Broeckling et al. (2008) when non-resident root exudates were added to resident plant treatments (Arabidopsis thaliana and Medicago truncatula), which the authors suggested could be attributed to increased production of signaling compounds in the roots triggered by the root exudates. Where invaders are selectively able to benefit from increased AMF colonization due to unique signaling compounds, they will experience advantages relative to proximal native plants, particularly in moisture- or P-limited environments.
Prevailing plant-fungal post invasion responses are listed in Table 1.
Table 1.
Alternative plant-fungal strategies of invasive plants in a novel range. Shifts in soil fungi will vary in the plant rhizosphere as invasion progresses, depending on the nature of the fungi introduced by or associating with the invader, and the root exudates of the invader. Native versus invader responses are suggested.
|
Increase in AM fungal diversity and abundance
|
Decrease in AM fungal diversity and abundance
|
No change in fungal community
|
Main references
|
| Invader has caused fungal shift or brought novel AMF, increasing AMF diversity/abundance, potentially resulting in increased nutrient uptake or pathogen protection<br/> Natives may benefit if host specificity is either suitable or not a factor |
Invader introduced a novel AMF species into the soil environment that is unable to colonize natives, but becomes pervasive<br/> Invader sustains decreased AMF colonization density or richness, while simultaneously increasing potential fungal pathogen load in rhizosphere |
Invader is able to form associations with existing AMF but experiences escape from resident pathogens in novel environment<br/> Invader is a fungal generalist that associates with a variety of local AMF without causing significant disruption or change to fungal community |
Appoloni et al. 2008, Bastias et al. 2007, Batten et al. 2006, Beckstead and Parker 2003, Callaway et al. 2003, Graham and Eissenstat 1994, Hawkes et al. 2006, Keene and Crawley 2002, Meinhardt and Gehring 2012, Mogg et al. 2008, Moora et al. 2011, Mummey and Rillig 2006, Richardson et al. 2000b, Van Aarle et al. 2009
|
| Increased signaling compounds (root exudates) added to rhizosphere by Invader enables increased AM and general fungal colonization<br/> Benefits to natives vary |
Invader releases toxins that decrease general and AM fungal persistence in the rhizosphere; allelopathy; possibility of decreased pathogen protection and/or nutrient/moisture uptake in proximal natives |
Invaders capitalizes on alternate strategies to dominate the system such as allelopathy, ability to compete in resource-depleted or disturbed environments more successfully than natives |
Antunes et al. 2008, Bais et al. 2003, Broeckling et al. 2008, Callaway et al. 2004, Callaway et al. 2008, Douglass 2008, Parniske 2008, Pozo and Azćon-Aguilar 2007, Stinson et al. 2006
|
| Reduced fungal pathogens occupying root infection loci due to infusion of novel chemicals by Invader; AMF are able to take up residence in newly available loci of Invader (possibly natives) |
Invader is able to form associations with existing AMF via hyphal fragments or anastamoses, where natives are not and such AMF species become pervasive in the rhizosphere |
Increased fungal pathogens occupying infection loci cause reduced AMF colonization – net effect is decrease in symbionts but increase in commensalists and pathogens |
Biermann and Linderman 1983, Callaway et al. 2003, Daniell et al. 2001, Giovannetti et al. 1999, Helgason et al. 2002, Mogg et al. 2008, Vigo et al. 2000
|
AMF and invader root exudate interaction studies
Although most authors have focused their research on either the benefits/anti-pathogenic properties of AMF or the benefits associated with invader plant root exudates independently, there are some examples of much needed interaction studies. Newsham et al. (1994) explored the relative effects ascribed to both AMF and fungal pathogens (potentially controlled by either AMF or root exudates) in the field, and determined how the two groups of fungi interacted to determine plant response and fitness. They found that while AMF colonization of winter annual grass Vulpia ciliata ssp. ambigua was reduced by the application of anti-fungicides, the root, shoot, total plant biomass, and phosphorus inflows were unaffected. The authors hypothesized that this somewhat unexpected finding arose due to the fungicidal depression of pathogenic root-inhibiting fungi such as Fusarium oxysporum or Embellisia chlamydospora isolated in the roots of Vulpia ciliata that might otherwise be compromising plant growth and fecundity. Newsham et al. (1994) concluded by suggesting that the main benefit supplied by arbuscular mycorrhizal fungi to the plant was apparently protection from pathogenic attack, not phosphorus uptake. Significant reductions in fecundity resulted from the root pathogen associations in Vulpia ciliata, though the infections were otherwise asymptomatic, presenting a specialized case of plant-pathogen interaction. The authors postulated that there was an interaction effect between the AMF and pathogenic fungi on some level, resulting in the ostensibly asymptomatic nature of the infection by pathogens. Observations of both sets of fungi in an invaded environment provide insights as to simultaneous shifts that could be instigated by exposure to invader root exudates or alterations to AMF initiated by the invader via diverse strategies.
In a study by Klironomos (2002), local fungal pathogens demonstrated a negative impact on growth and spread of rare plants when grown in local or home soil over time, while invasive plants demonstrated net positive growth when grown in their own local soil (and associated fungi), suggesting reduced susceptibility to local pathogens over time. The effects of AMF, however, did not differ between the rare and invasive plant groups. For both groups (native and invaders), AMF isolated from soil with a history of the same plant species had a more positive effect on plant growth than fungi isolated from a different plant host. With both groups of plants, inoculations using AM fungi from foreign plants rarely resulted in significant growth depressions. These findings suggest that most plants can potentially experience positive feedback with AMF communities, but this effect is not realized unless negative feedback resulting from pathogens is limited. This arises as a consequence of the differential effect of pathogenic fungi on invasive plants relative to rare plants, rather than a differential response to the AMF. When introduced to previously uncultured soil at low densities, plants that ultimately achieve high abundance do not seem to accumulate species-specific pathogens at the same rate as plants that remain in low abundance (Klironomos 2002). Pathogen accumulation in highly successful invasive plant species tends to happen more slowly than that of natives demonstrating low abundances due to negative feedback mechanisms, including dense colonization by microbial pathogens (Bever 2002, Klironomos 2002, Mitchell and Power 2003, Inderjit and van der Putten 2010).
Studies investigating simultaneous shifts in both the general fungal community and AMF would provide clarification as to the role of each subsequent to plant invasion. In doing so, they would elucidate the combined mechanisms of invader root exudates invoking pathogen reduction via allelopathy, as well as the response of altered or introduced AMF subsequent to invasion. While other interaction study methods have been suggested, including use of microbial microarrays and detailed assays of root exudates to differentiate phytochemicals that signal AMF hyphenation and infection from those targeting disruption of rhizosphere pathogens (Bais et al. 2006), the use of molecular alternatives such as T-RFLP and other fingerprinting methods could provide the same result at a reduced effort. Primers targeting the general fungal community differentially from primers targeting AMF produce both a proxy for the rhizosphere response to allelopathic chemicals in invaders roots (shifts in general fungal community), as well as directly observable shifts in the AMF community.
Conclusions and future directions
It is necessary to design experiments that can account for the multiplicity of events taking place in local soil environments post invasion to gain a greater understanding of the fungal-chemical interactions in the rhizosphere (local scale), and within the ecosystem (regional scale) as the plant invasion progresses. Investigating fungal communities (both general and AMF) in pre- and post-invaded sites over time would be useful in determining how each factor, such as the state of AMF or non-AM fungi (comprised of fungal pathogens, commensalists, and mutualists), as well as the influence of invader root exudates or the interaction of all of thein both general and AM fungal communities, with the variable TRFs serving as a proxy for different fungal phylotypes associating with the plant groups. Where TRF data show a tendency for the general fungi associating with allelopathic plants to differ from the non-allelopathic plants in both natives and invaders, but not similarly for the AMF community, allelopathy is then recognized as a factor rather than the various scenarios linked to benefits associated with increased or altered AMF post invasion. Where pathogenic fungi are being depressed or are well tolerated in invader plant roots but AM fungi are not similarly altered, it suggests that natives growing in an invaded patch may be susceptible to higher pathogen loads relative to natives that are unexposed, supporting the hypotheses of both Eppinga et al. (2006) and Klironomos (2002). Alternatively, natives may be more negatively affected by specific pathogen introductions associated with an invader than the invader itself. This effect will likely vary considerably for different native plant species, and individual investigations that are relevant to the invasion site would be informative. These types of molecular investigations also allow for comparisons of AMF diversity and density changes relative to those occurring in the general fungal community (including pathogens) as invasions progress. It would be expected that the general fungal community would be less diverse/abundant in invader roots relative to natives if pathogenic fungi are being depressed or virtually eliminated by phytochemicals (root exudates) in invaders that are not present in natives. As well, looking at the response of AM fungi relative to responses in the general fungal community would also potentially reveal the relative role of AMF as invasion progresses (Hawkes et al. 2006, Mummey and Rillig 2006).
In considering alteration of soil fungal communities by invasive plants, differential fungal association dynamics need to be quantified and compared, a process that is well supported by molecular investigation methods. Comparing both AMF and general fungal community fingerprints within a variety of native plant roots in pre- and post-invaded sites will provide insight as to whether shifts in AMF or the general fungal community (or both) are facilitating the invasion process. Molecular fingerprinting techniques could also be used to investigate variation in the diversity and density of fungal colonization during different stages of invader succession to determine which species are supporting the establishment and progression of invaders, particularly in light of the fact that delayed proliferation in time and space (due to Allee effect) has been observed in many invasive plant species.