(C) 2012 Heidi Hirsch. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Germination is a crucial step for invasive plants to extend their distribution under different environmental conditions in a new range. Therefore, information on germination characteristics of invasive plant species provides invaluable knowledge about the factors which might contribute to the invasion success. Moreover, intra-specific comparisons under controlled conditions will show if different responses between non-native and native populations are caused by evolutionary changes or by phenotypic plasticity towards different environmental influences.
This paper focuses on the germination of native and non-native Ulmus pumila populations. We expected that non-native populations would be characterized by their higher final germination percentage and enhanced germination rate, which might indicate an influence due to corresponding climatic conditions.
Germination experiments with a moderate and a warm temperature treatment did not reveal significant differences in final germination percentage. However, seeds from the North American non-native range germinated significantly faster than native seeds (p < 0.001). Additionally, mean time to germination in both ranges was significantly negatively correlated with annual precipitation (p = 0.022). At the same time, this relationship is stronger in the native range whereas mean time to germination in non-native populations seems to be less influenced by climatic conditions.
Different germination responses of the North American populations could be caused by a fast evolutionary change mediating a higher tolerance to current climatic conditions in the non-native range. However, our findings could also be caused by artificial selection during the introduction process and extensive planting of Ulmus pumila in its non-native range. Nevertheless, we assume that the faster germination rate of non-native populations is one potential explanation for the invasion success of Ulmus pumila in its new range since it might provide a competitive advantage during colonization of new sites.
Climatic influence, survival analysis, biological invasions, Ulmus pumila
Introduced species often face different environmental conditions in their new range compared to their range of origin. Therefore, non-native species have to overcome several factors before they can become invasive (
Differing germination characteristics can be caused by evolutionary changes mediated by corresponding environmental conditions. For example,
Intra-specific comparisons between native and non-native populations are important to understand the mechanisms of the invasion process (
Although more than 300 tree species are classified as invasive, there are comparatively few studies on their invasion success (
We focused our study on non-native populations in the Western U.S. and compared their germination performance under controlled conditions to the performance of populations from the native range in China. Thereby, we tested the following hypotheses: 1) Non-native populations will exhibit an increased percentage of germinated seeds. 2) Non-native populations are characterized by a faster germination. 3) Different germination responses might be influenced by different climatic conditions. In this context, we assume that populations located in regions with less stressful climatic conditions (e.g. higher annual precipitation) show enhanced germination characteristics.
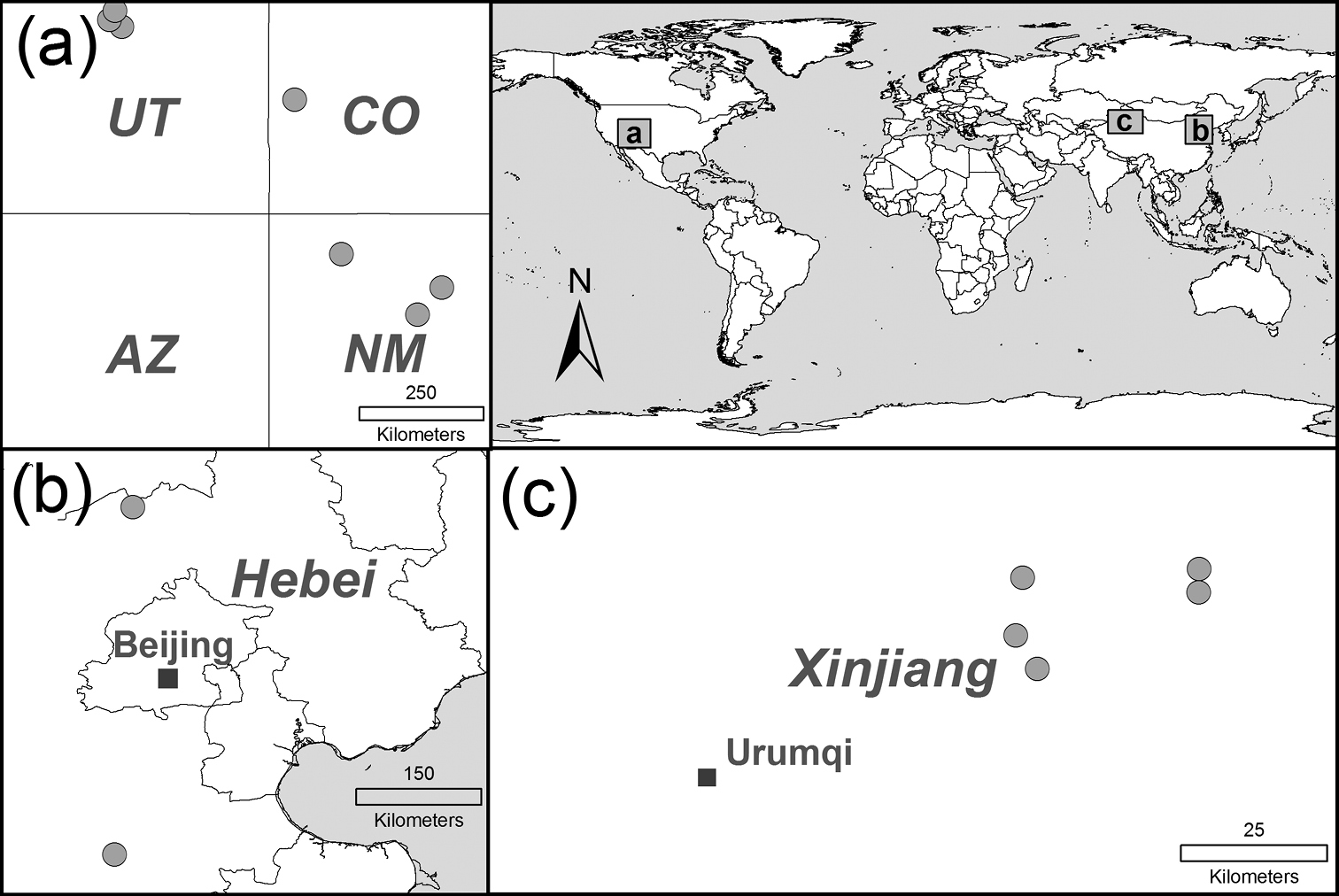
We retrieved samaras (henceforth referred to as seeds) from seven populations from the native range (China) and seven populations from the non-native range (U.S.; Figure 1). Seeds from China were collected in May and June 2009 and seeds from the U.S. in May and June 2010. We sampled at least 15 trees per population and pooled the seeds within populations. Where seeds had already been shed, they were collected from the ground. Material was stored in sealed plastic bags at 4°C following recommendations by
Sampled Ulmus pumila populations in the native range (b, c) and the non-native range (U.S.: a; AZ = Arizona, CO = Colorado, NM = New Mexico, UT = Utah). Populations are indicated by gray circles.
The germination experiment started in January, 2011 and was setup as a completely randomized design with eight replicates per population and treatment. Each replicate contained 20 seeds which were placed on filter paper in standard Petri dishes. In sum, we used 4480 seeds (14 populations × 2 temperature treatments × 8 replicates × 20 seeds per replicate) in our experiment. The dishes were filled with de-ionized water to keep the seeds permanently moist. Wings of the seeds were not removed due to their role in facilitating water uptake and in order to avoid seed damage (
All statistics were calculated with the software R (version 2.15.0;
To test if the mean time to germination is adapted to different climate conditions between the native and the non-native range (see Appendix 2: Figure A1), we extracted climatic information per population (mean annual temperature and annual precipitation; see Appendix 1: Table A1) from the WORLDCLIM database (
At the end of the germination experiment, 80.6 % of the tested seeds were germinated. From the non-germinated seeds were 2.1 % still viable (non-native origin: 1.3 %; native origin: 0.8 %).
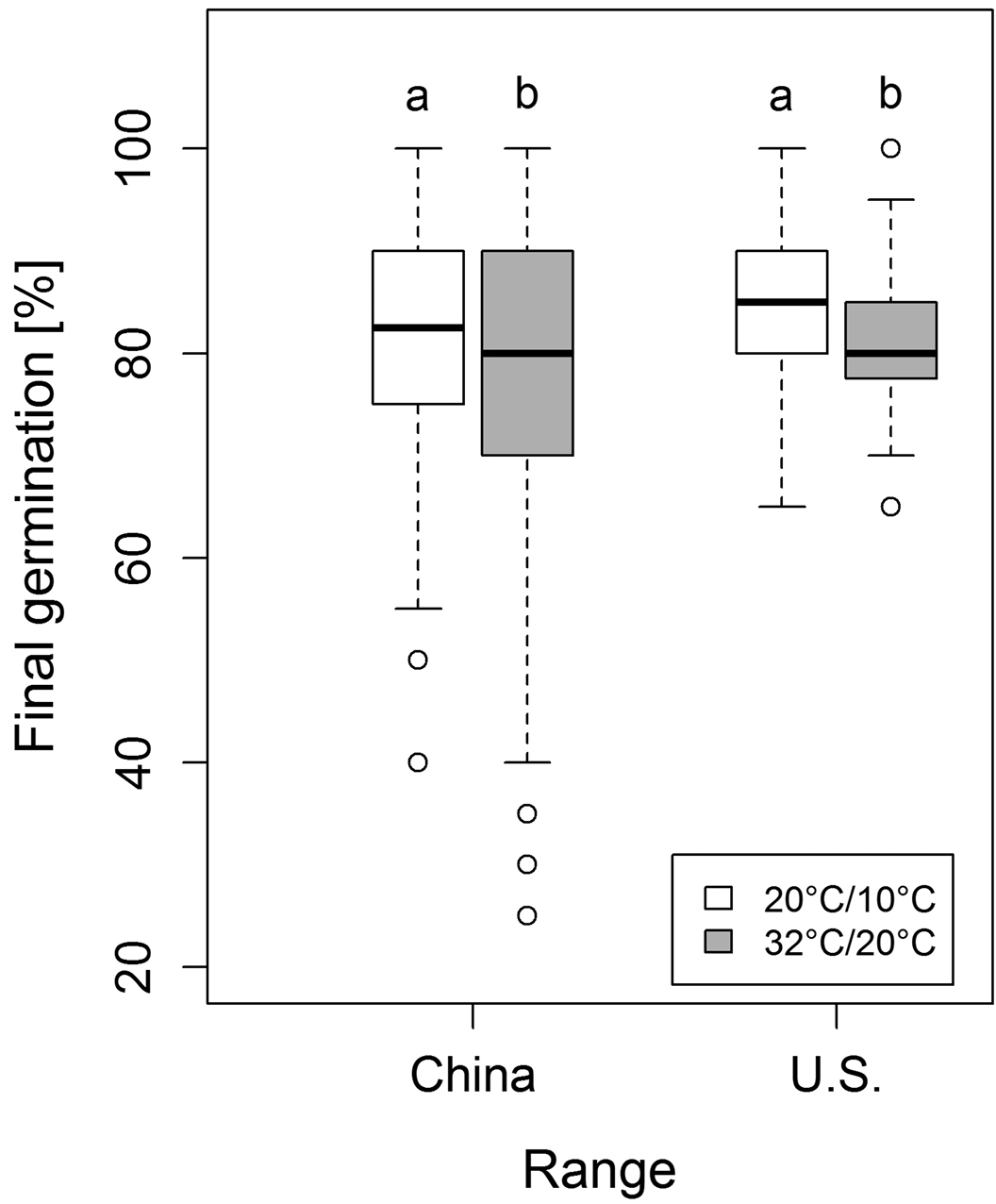
The test for differences regarding the final germination percentage resulted in a final model containing only the temperature treatment as fixed effect. Consequently, no differences between the two ranges were detectable (F1, 12 = 0.416, p > 0.05, Figure 2). However, final germination percentages for both ranges were slightly lower under warm temperature conditions compared to a moderate temperature (F1, 209 = 6.513, p < 0.05; Figure 2).
Final germination (%) of Ulmus pumila seeds from native and the non-native ranges. No differences were found between the two ranges (F1, 12 = 0.416, p > 0.05). Germination percentage was significantly decreased under warmer temperature treatment (F1, 209 = 6.513, p < 0.05; significant differences are shown by different letters above the boxes).
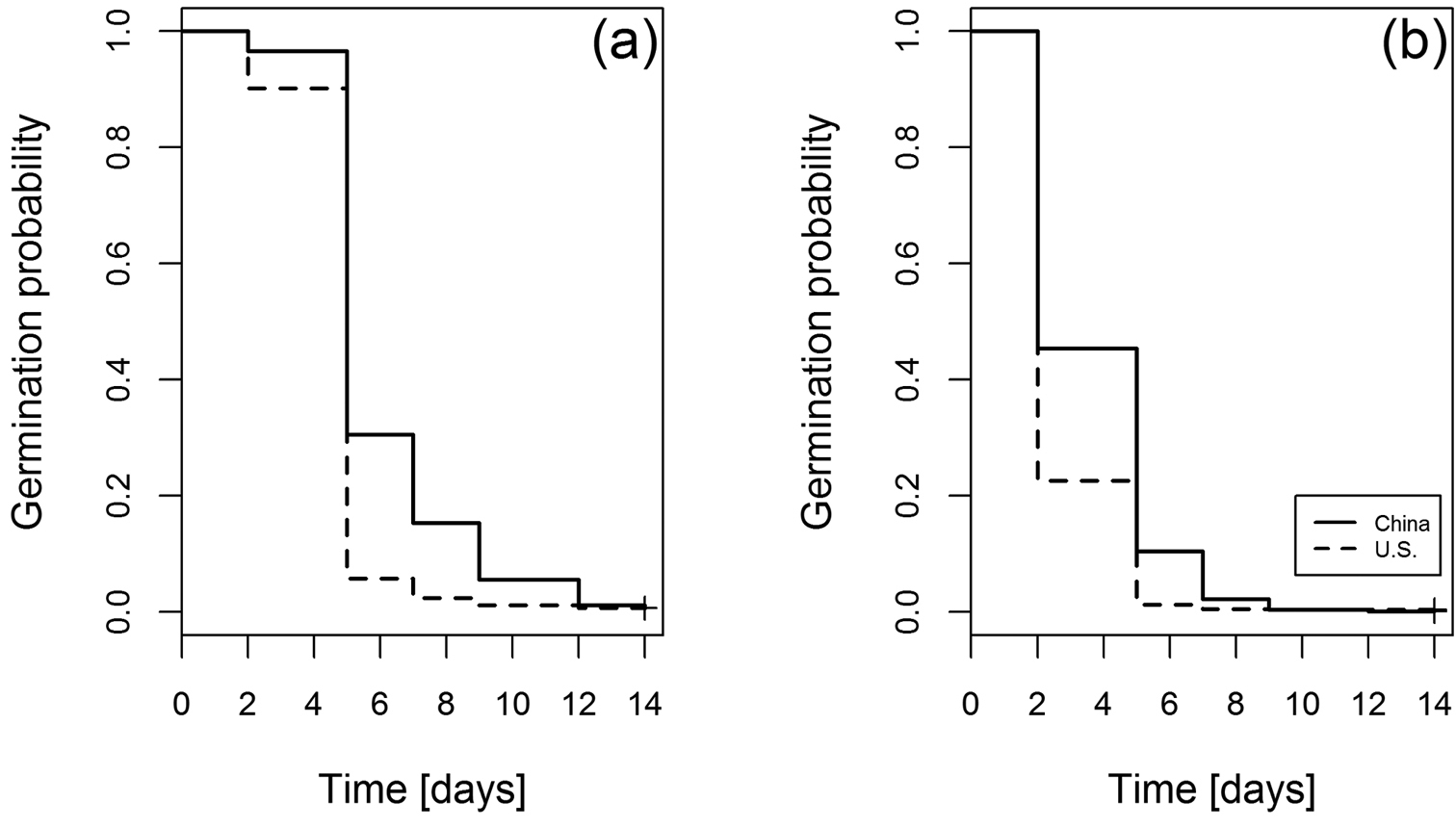
Investigation of the time to germination revealed that the most pronounced reduction of deviance was contributed by the temperature treatment (Table 1). Lower, but still significant effects were contributed by the random effect (population), the influence of the origin of the populations (range) as well as the interaction between range and temperature treatment. These results were obtained from a final AFT model with best fit for log-normal distribution showed including range as well as temperature as predictor variables and population as random effect. The enhanced germination rates under warmer temperatures as well as the differences between the two ranges are visualized in Figure 3.
Analysis of deviance results for the AFT model. The results show the differences in the time to germination of Ulmus pumila under consideration of range and temperature treatment (df = degrees of freedom).
| Source | df | Deviance | Residual df | -2 × loglikelihood | p |
|---|---|---|---|---|---|
| Null model | 3627 | 15151.90 | |||
| Range | 1 | 165.75 | 3626 | 14986.25 | <0.001 |
| Temperature | 1 | 1691.68 | 3625 | 13294.47 | <0.001 |
| Frailty (population in range) | 12 | 673.16 | 3615 | 12621.30 | <0.001 |
| Range × temperature | 1 | 6.34 | 3614 | 12614.96 | 0.012 |
Kaplan-Meyer curves of the germination functions for the non-native and native origins of Ulmus pumila. Curves are shown for the two temperature treatments (a: 20°C/10°C; b: 32°C/20°C). Censored data is symbolized by final crosses at the curves. The curves show the probability that seeds will germinate. Therefore, the germination probability has to be 1.0 at time = 0 because all seeds are non-germinated and have consequently the chance to germinate.
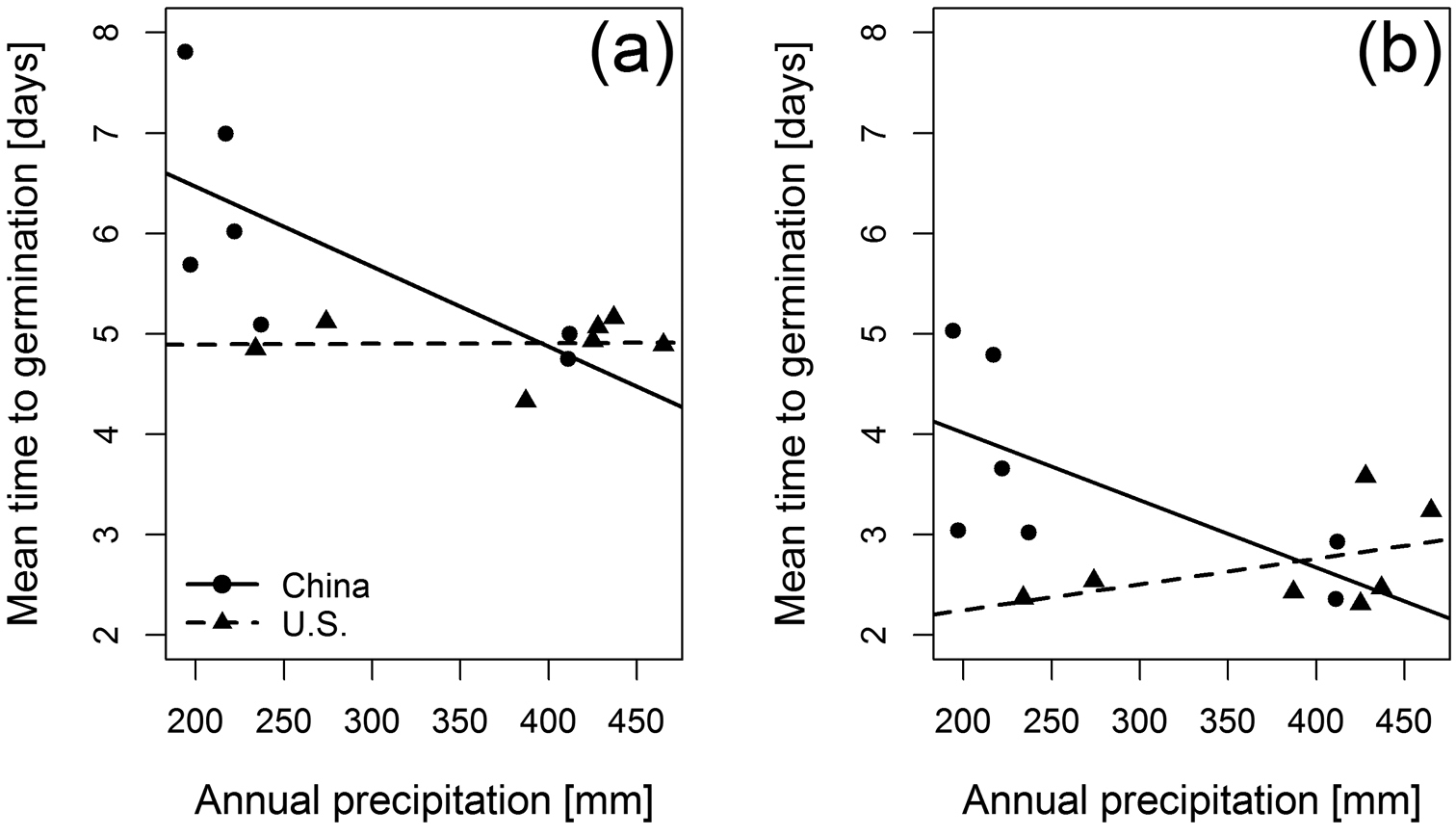
Considering the mean time to germination supports our result of the AFT model regarding a faster germination at higher temperatures (F1, 23 = 88.83, p < 0.001) and in the non-native range (F1, 23 = 14.48, p = 0.001). We also found a significant negative relation between mean time to germination and annual precipitation (F1, 23 = 5.98, p = 0.022) as well as a significant interaction between range and annual precipitation (F1, 23 = 9.46, p = 0.005). This interaction shows that native populations with less annual precipitation are characterized by increased mean times to germinate. In contrast, non-native populations show only weak response in their mean time to germination towards corresponding annual precipitation conditions (Figure 4). These results were obtained from the multiple regression model retaining population origin, temperature and annual precipitation as predictor variables after stepwise model selection (multiple R² = 0.84, p < 0.001).
Relationship between mean time to germination and annual precipitation per Ulmus pumila population (a: 20°C/10°C; b: 32°C/20°C). To emphasize the different responses between the two ranges (non-native range: triangles; native range: circles), trend lines per range are shown (non-native range: dashed line; native range: solid line).
Our results revealed a slightly lower final germination of Ulmus pumila seeds at higher temperatures. This could be caused by an earlier and stronger infestation by mold fungi at the 30°C/20°C temperature treatment (personal observation), because these warmer temperatures provide better growing conditions of mold fungi. For example,
Contrary to our hypothesis, the invasion success of Ulmus pumila in North America does not seem to be based on an enhanced final germination percentage. However, we have evidence for enhanced times to germination in non-native populations. We propose that the fast germination is one of the contributing drivers for the invasion success of Ulmus pumila because it could provide advantages during inter-specific competition in the colonization processes (
Additionally, we found that mean time to germination in both ranges seems to be influenced by climatic conditions such as annual precipitation (i.e. mean time to germination decreases with increasing annual precipitation). We assume that this relationship is based on less stressful germination conditions for the Siberian elm under climatic conditions with more rainfall since annual precipitation can be considered as a general measure of environmental quality (
In contrast to natural evolutionary processes, the pattern of different germination reactions in our studied populations could also be caused by human-mediated selection of successful lineages during introduction (
Additionally, it should be considered that our results could be biased by two methodical factors. First, differences between both ranges might be caused by different sampling years (seeds from the native range: 2009; seeds from the non-native range: 2010). We assume that this factor induced only a negligible influence to our results, because
It should also be mentioned that several other studies have shown that changed germination characteristics are often linked to changed post-germination traits as well (
Our work suggests that changed germination characteristics could be one of the drivers for the invasion success of Ulmus pumila. However, further research (i.e. genetic analyses and growth experiments) is needed to find genetic evidence for our assumption and if the assumed evolutionary change of germination responses also influenced other early life cycle traits of non-native populations of the Siberian elm.
We thank A. Schneider, S. Hegi, R. Peterson, R. Sivinski, J. Smith, T. Frates and B. King who pointed us to populations and provided hospitality during the field trip in the U.S. Our thanks go also to Ximing Zhang, Zhenying Huang, B. Oyuntsetseg and R. Suarez for collecting and sending sampling material of Ulmus pumila. For invaluable help during the experiment, we thank C. Voigt and M. Hartmann. We also thank C. Rosche for helpful comments on the manuscript. We thank J. Zalapa and E. Gustin for their help editing this manuscript and the anonymous reviewers for their valuable comments which helped to improve this manuscript significantly. This study was funded by the “Graduiertenförderung des Landes Sachsen-Anhalt” and by the DAAD.
Table A1: Location and climate information of the sampled Ulmus pumila populations. (doi: 10.3897/neobiota.15.4057.app1) File format: PDF.
Explanation note: Location and climate information of the sampled Ulmus pumila populations in China and the U.S. Maximum (max.) temperatures for the months May, June and July are provided to show the temperature range during the main germination period (lowest and highest values are italicized). Climatic information was extracted from the WORLDCLIM database (
Figure A1: Comparison of climatic conditions between the Chinese and North American locations of Ulmus pumila. (doi: 10.3897/neobiota.15.4057.app2) File format: PDF.
Explanation note: Comparison of climatic conditions (a: mean annual temperature; b: annual precipitation) between the Chinese and North American locations of Ulmus pumila. Wilcoxon rank sum tests were used to test for differences between both ranges. Mean annual temperatures are significantly higher for locations from the U.S. (W = 7, p < 0.05). Annual precipitation is marginal higher in the invasive populations compared to the native populations (W = 9, p = 0.05). Significant differences are symbolized by different lowercases above the boxes.