(C) 2013 Ryan D. Spafford. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Invasive plants represent a significant financial burden for land managers and also have the potential to severely degrade ecosystems. Arthropods interact strongly with plants, relying on them for food, shelter, and as nurseries for their young. For these reasons, the impacts of plant invasions are likely strongly reflected by arthropod community dynamics including diversity and abundances. A systematic review was conducted to ascertain the state of the literature with respect to plant invaders and their associated arthropod communities. We found that the majority of studies did not biogeographically contrast arthropod community dynamics from both the home and away ranges and that studies were typically narrow in scope, focusing only on the herbivore feeding guild, rather than assessing two or more trophic levels. Importantly, relative arthropod richness was significantly reduced on invasive plant species. Phylogenetic differences between the invasive and local plant community as well as the plant functional group impact arthropod diversity patterns. A framework highlighting some interaction mechanisms between multiple arthropod trophic levels and native and invasive plants is discussed and future research directions relating to these interactions and the findings herein are proposed.
Arthropod, invasive plant, multi-trophic interactions, biogeographic contrast, phylogenetic differences
Invasion is a worldwide epiphenomenon as a consequence of both significant dispersal and global change, and the environmental costs are staggering (
The ecological research on native herbivore effects on invasive plants is equivocal depending on the herbivore species, plant taxa, and spatial and temporal context (
We propose that a powerful evaluation of plant invasion processes can be achieved by documenting whole arthropod community dynamics (e.g. richness, diversity, interactions) in the native and introduced range of a plant invader. Biogeographically contrasting invasion dynamics is rarely practiced (
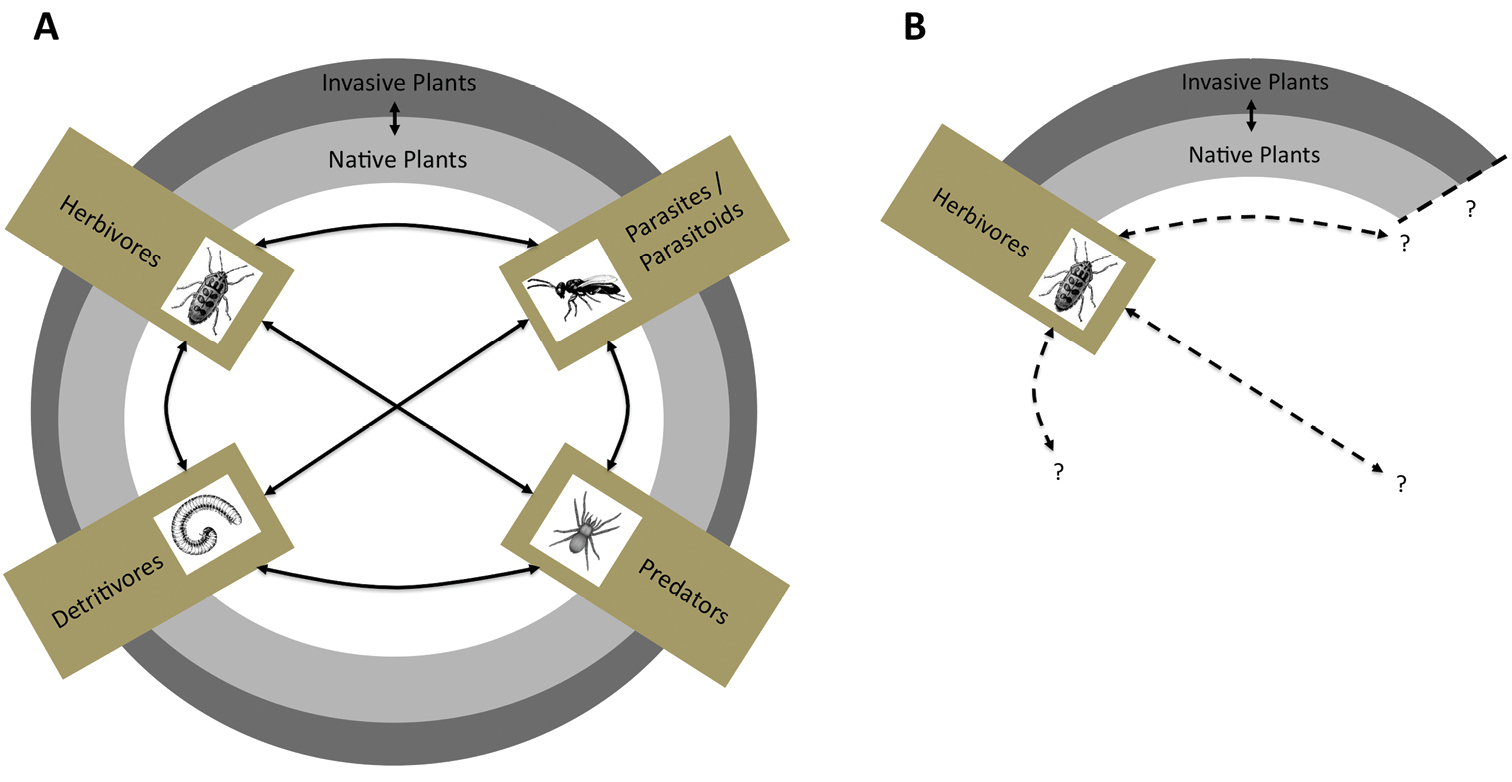
A conceptual framework of potential interactions in native/invasive plant-arthropod systems. Herbivores, predators, parasitoids, and detritivores are all linked to native and invasive plant community complexes (boxes embedded within concentric native/invasive plant circles). Solid lines denote reciprocal interactions between arthropod feeding guilds B Dashed lines denote the uncertainty introduced when only herbivores are targeted in plant invasion studies. The influence of multi-trophic interactions becomes lost when studies of plant-arthropod systems are limited in scope to only the herbivorous feeding guild.
The purpose of this systematic review was to quantify the state of knowledge of arthropod community dynamics in the context of plant invasion, in order to examine the general hypothesis that a biogeographical and multi-trophic examination of arthropod communities enhances evaluations of plant invasions. Specifically, we explored whether: (1) biogeographical contrasts of the arthropod communities associated with invasive plants are under-utilized in the invasion biology literature; (2) arthropod sampling is biased to the herbivore feeding guild and largely ignores the arthropod community as a whole; (3) relative richness of arthropods associated with invasive plants is lower than commonly found on native plants; and (4) phylogenetic differences between the invasive plant and the local plant community and the plant functional group of the invader have the capacity to impact arthropod diversity. Exploration of the literature via quantitative systematic review provides a broad assessment of the importance of local arthropod communities as an indicator or even predictor of invasive plant species dynamics, and studies documenting the dynamics of entire arthropod communities are a logical step in future evaluations of plant invasions.
A systematic review of the literature using the Web of Science was conducted in September 2011 using following keywords: “invas* plant* (insect OR arthropod OR herbivor* OR natural OR phytophag*) and (diversit* OR abundance OR richness OR herbivory OR removal OR enem*)”. A total of 1746 studies were retrieved. However, studies were retained for this review only if they explicitly included arthropods, i.e., studies on mammals were excluded. Aquatic systems and secondary studies not based on experimental data directly collected by the authors (i.e. review or idea articles) were also excluded. Finally, all references cited within these articles were also inspected and included to further extend scope.
A total of 53 relevant articles published in 31 different journals were selected for inclusion in this review. The first study was published in 1982, and only three studies were published prior to 2000. The majority of studies (38%) were published in 2009 and 2010. These articles have been cited a total of 759 times as of December 2011. The number of citations/article ranged from 0 to 104 (0 to 14.86 citations/year; mean = 2.23), with most articles (70%) being cited less than 10 times, indicating that perhaps literature corresponding to arthropod community dynamics on invasive plants is not highly visible. Journals contributing the highest number of articles were Biological Invasions (17%), Biological Conservation (9%), and Environmental Entomology (7%).
To characterize the literature on native arthropod communities associated with non-native plant invasions, the following parameters of each study were recorded: ecosystem type (e.g. grassland, experimental field, waste area); the country in which it took place and whether or not it was biogeographical (i.e. data on arthropod communities in association with the invasive recorded in more than one region); native plant species community richness; invasive plant species taxonomy; the plant functional group (PFG) of each invader (tree, shrub, graminoid, or herb); native arthropod community characteristics on invasive host plants/within invaded habitats (i.e. abundance, order, family, and species level richness) and; the class and number of arthropod trophic levels examined (i.e. herbivores, predators, detritivores). Studies were permitted more than one database entry if they examined more than one non-native plant species or geographic region. As this study is strictly a systematic review and not a meta-analysis, effect sizes were not calculated.
Descriptive statistics were used to explore the first two broad patterns associated with the literature including Chi-square tests for differences in relative proportion of studies where appropriate. Generalized linear models (GLMs) were used to explore the latter two patterns that diversity of arthropods is affected by native versus invasive plants and then by PFG and phylogenetic measures of these plants (firstly, we used the entire dataset and then did a second more direct test via paired t-tests of only the studies that used coupled contrasts). Alpha was set at p < 0.05, and post hoc contrasts were applied when significant to identify specific differences if more than two levels (Nonparametric Wilcoxon signed-rank tests were used as highly conservative between-level tests). Studies were included in these analyses if more than a single trophic group was examined, arthropod richness estimates were provided, and contrasts between target (i.e. on the invasive plant) and native plants or within the community were reported in some form. A total of 4 studies reported only order-level arthropod richness whilst all others reported species-level estimates. The order-level values fell within one standard error of the mean of species-levels estimates so were not excluded. The log response ratio (LRR) was also calculated to summarize the strength of the relative difference between arthropods associated with native versus invasive plants (
Finally, phylogenetic relationships among all 1045 plant species reported were constructed by grafting published phylogenies onto a family-level backbone based on the APG3 derived megatree produced with Phylomatic (
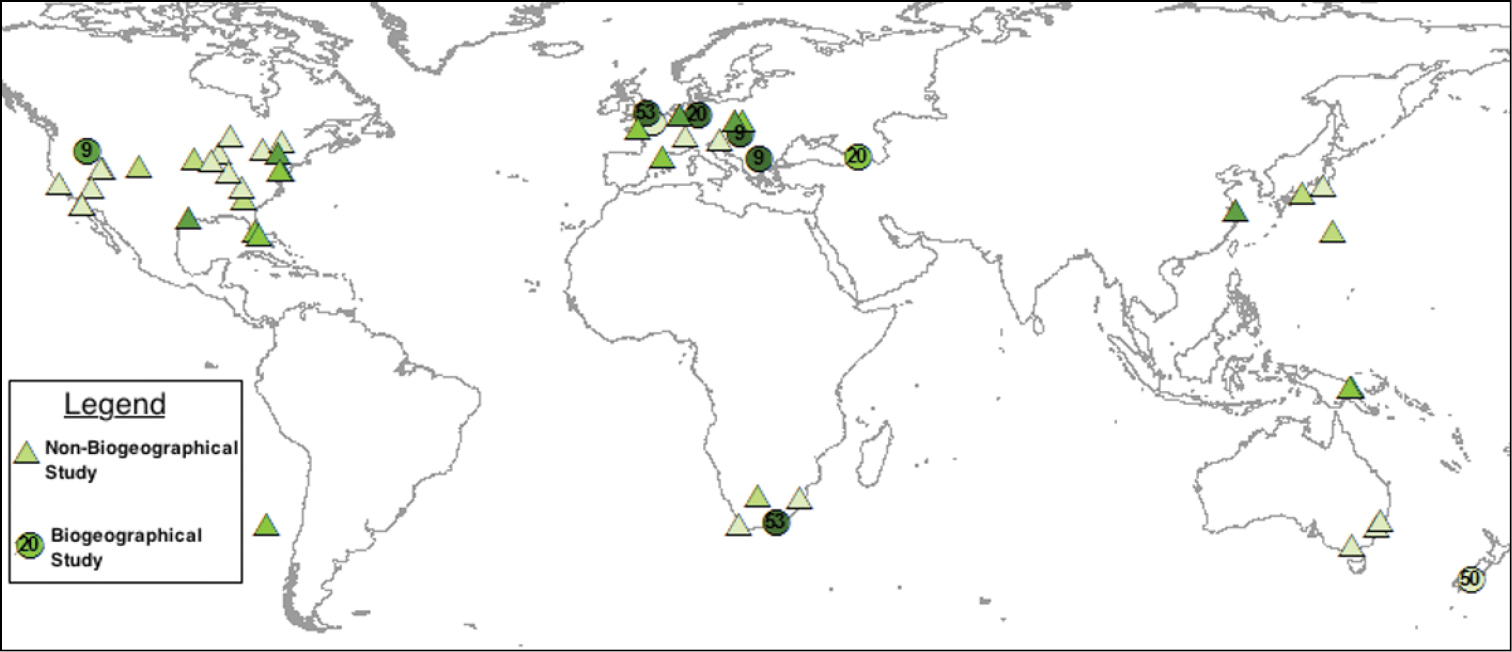
From the 53 studies included in this review 11 ecosystem types were censused for arthropods. In decreasing order of prevalence these were: grassland, mixed, forest, experimental field, marsh/wetland, shrubland, riparian, waste area, desert, dune, and floodplain ecosystems. Two studies did not detail the ecosystem from which data was collected. Geographically, arthropod communities were censused in 27 countries (Figure 2). Fifty three percent of all studies were conducted in North America, while 28% were conducted in Europe (Figure 2). Less than 8% of all studies (4/53) used biogeographical contrasts to record arthropod dynamics in the native and introduced ranges of invasive plant species.
A world map illustrating the geographic distribution of arthropod-invasive plant studies from the literature in this review. Darker coloured icons represent greater relative arthropod richness.
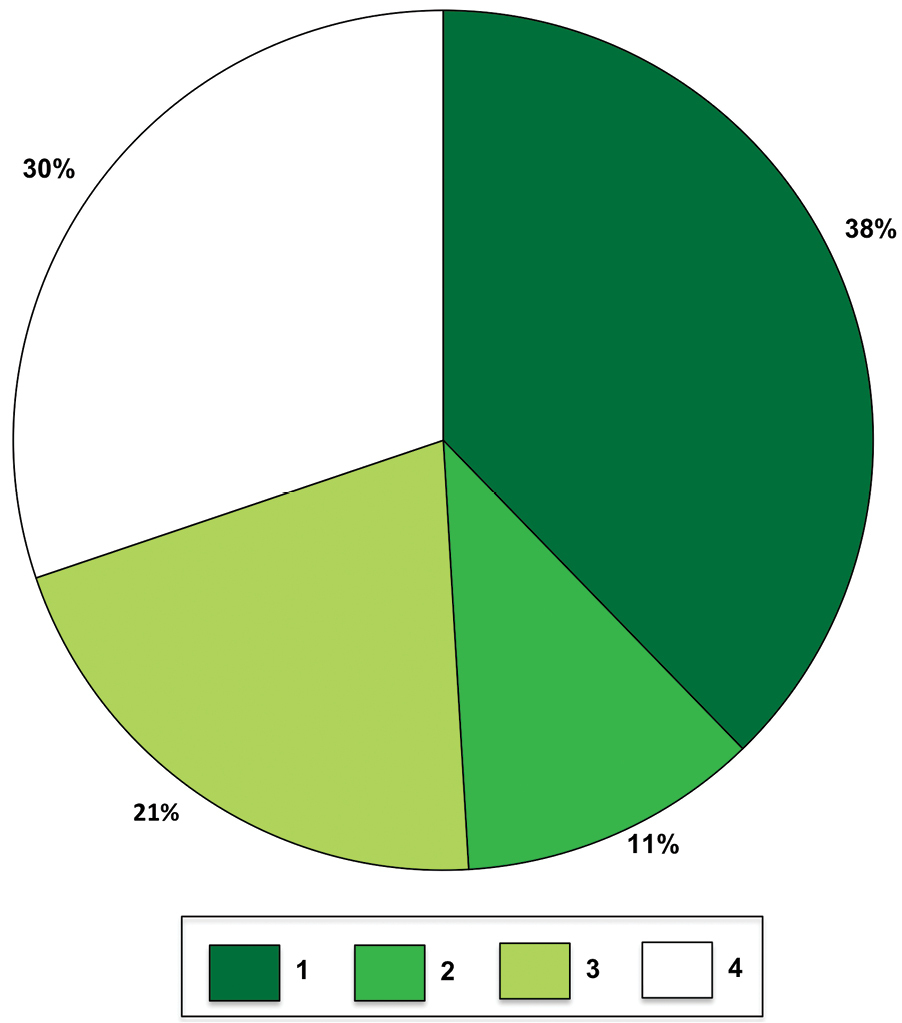
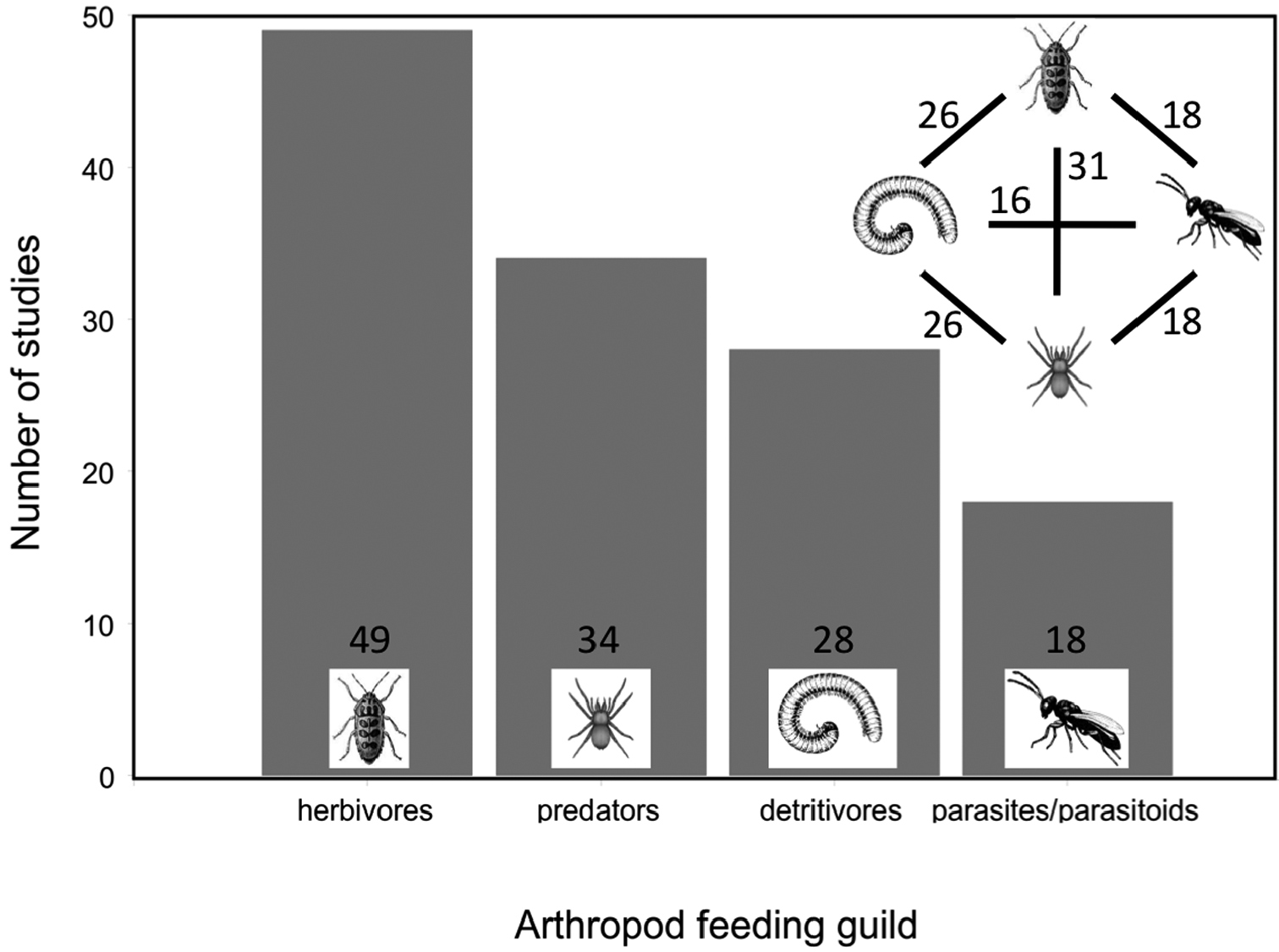
A total of 38% of studies measured only one trophic level whilst 30% of studies evaluated 4 trophic levels. Fewer studies evaluated only two or three trophic levels (Figure 3, 11% and 21%, respectively). These proportionate differences were significantly different (Chi-square, c2 = 8, p = 0.039, n = 53). A breakdown of studies based on which feeding guilds were examined indicated that the majority (92%) targeted at least herbivorous arthropods. Predators were measured in 64% of the studies, detritivores in 53%, and parasites/parasitoids in 34% (Figure 4, Chi-square, c2 = 16, p = 0.0013, n = 129).
Proportion of all 53 studies in this review examining either 1, 2, 3, or 4 trophic levels.
Number of all 53 studies in this review examining each of the arthropod feeding guilds.
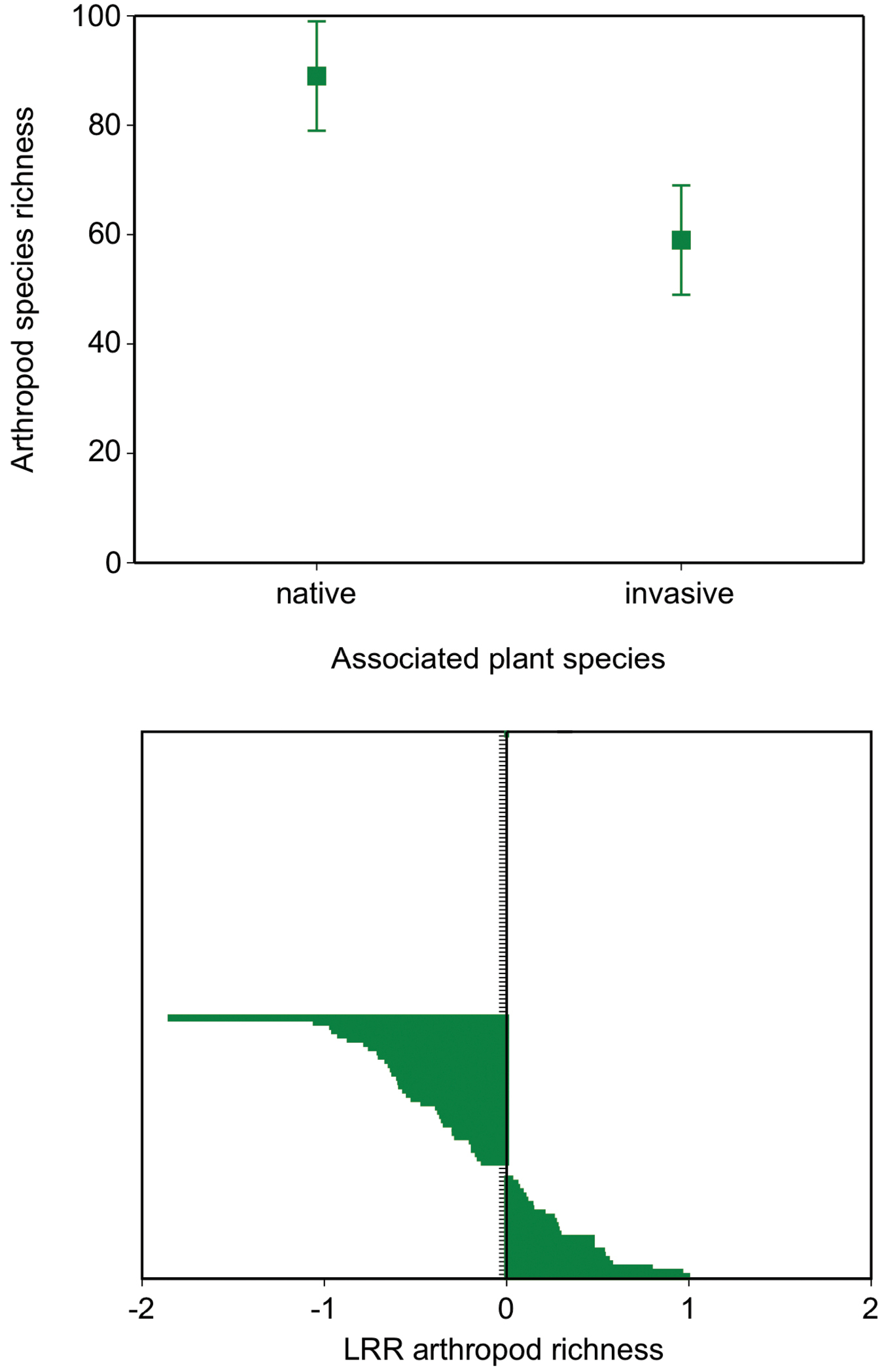
Arthropod richness estimates associated with invasive plants were significantly depressed relative to native plants or monoculture/community estimates using all cases (Figure 5, GLM, chi-square = 385, p = <0.0001, n = 124). Using only paired contrasts within studies, the strength of the relative depression in arthropod richness between invasive and native plants was -0.18 +/- 0.06 (mean LRR with s.e.), and this estimate was significantly different from a null of 0, i.e. no difference (t-test for mean diff from 0, t = -2.5, p= 0.01, n = 62 cases).
The diversity of arthropods associated with native and invasive plant species. The top plot shows the mean number of arthropod species reported on invasive target plants and the native community +/- 1 s.e. for all studies. The lower modified Forest plot shows the log response ratio (LRR) for only studies that used direct paired contrasts between an invasive and target plant species (n = 62 cases, see text for details). Negative values denote a relative reduction in arthropod species richness on invasives relative to native plants.
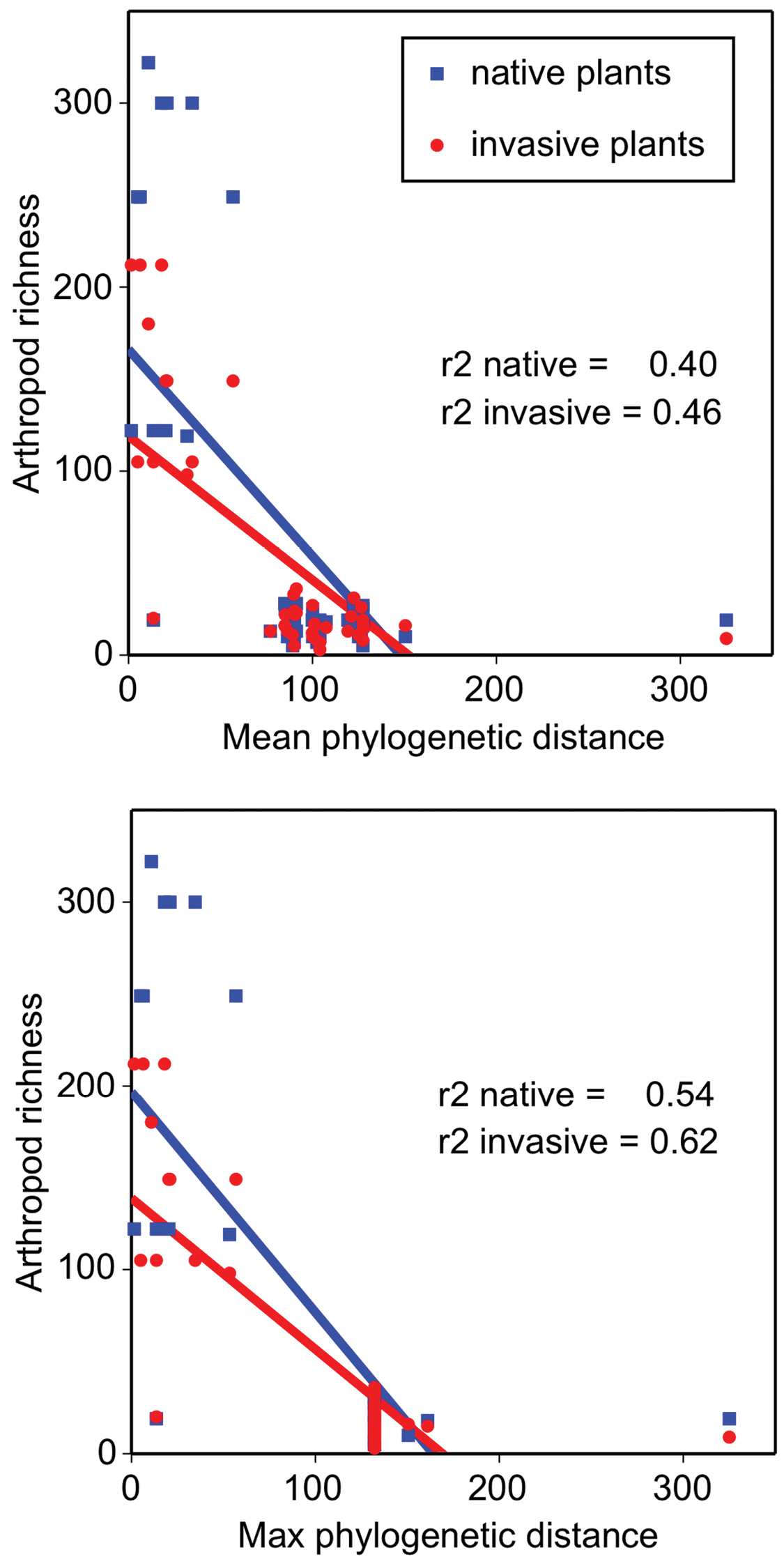
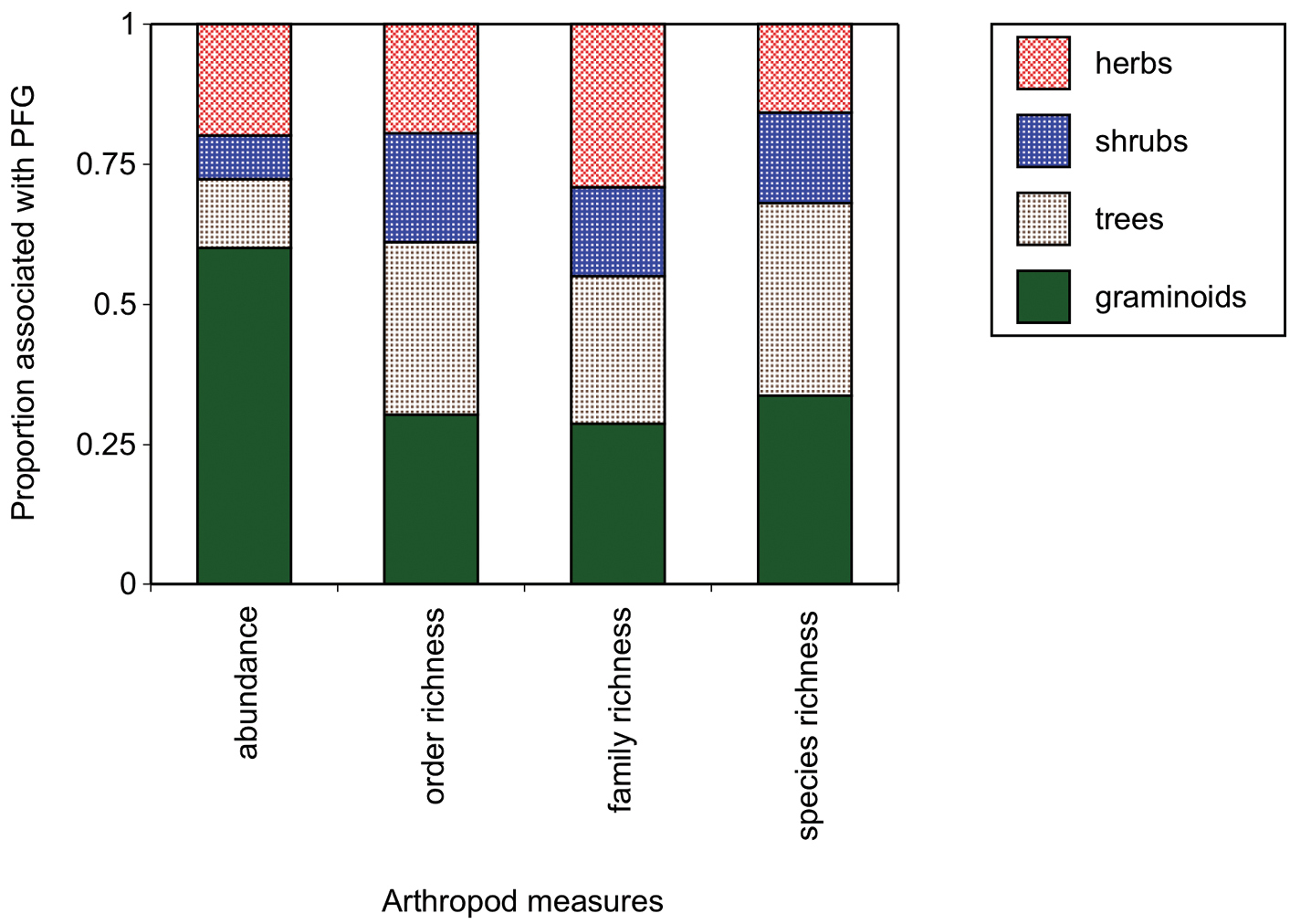
Both mean and maximum phylogenetic distances significantly predicted arthropod richness on invasive and on native plants (Figure 6, Regression analyses, all p < 0.0001, r2 values listed on plots). Increasing phylogenetic distances reduced arthropod diversity (Figure 6). Plant functional group significantly influenced arthropod richness at the species level (GLM, Chi-square = 33.8, p = 0.001, df = 1) but only for arthropods associated with invasive plant hosts - not native plants (GLM, Chi-square PFG*host= 80.3, p = 0.0001, df = 3 with Wilcoxon post hoc paired contrasts, p > 0.05 for all natives). Specifically, arthropod species richness differed between invasive trees and herbs (Figure 7, Wilcoxon paired contrasts, p = 0.02), and the abundance of arthropods associated with invasive trees differed from graminoids (Wilcoxon paired contrast, p = 0.05). Given the exploratory nature of this review, corrections for multiple comparisons were not made (
The effect of mean and maximum phylogenetic distance estimates on arthropod species richness on invasive and native plants. Linear regressions are shown (p < 0.0001).
The relative arthropod order richness, family richness, species richness, and abundance across PFG on invasive plant hosts. For simplification, relative proportions are plotted instead of raw data as values ranged widely.
The primary objective of this systematic review was to quantify the state of knowledge of arthropod community dynamics in the context of plant invasion. Results of this review highlight some key trends in the arthropod-invasive plant literature: few studies adopt a biogeographical approach when contrasting arthropod communities associated with invasive plants in both native and invasive ranges. Sampling is also relatively simple, primarily documenting only the herbivore feeding guild and not the arthropod community as a whole. The relative richness of arthropods associated with invasive plants is lower than commonly found on native plants suggesting direct or indirect depressions of arthropods. Finally, phylogeny and plant functional grouping can be important factors influencing these reductions in diversity. Arthropod communities clearly respond differently to invasive plants than to native plants.
Studying invasive species from a biogeographical perspective is a powerful yet underappreciated tool in invasion ecology (
Sampling regimes focusing on only the herbivore feeding guild comprise a large proportion of the literature (e.g. two of the four biogeographic contrasts in this review). While informative, these studies are not adequate to fully explain the mechanisms by which plant invaders are successful and may introduce uncertainty and thus false conclusions regarding observed declines in herbivores within invaded regions (i.e. Figure 1B). Herbivore declines are often attributed directly to invasive plants but they may be the product of an indirect interaction whereby an invader facilitates predacious or parasitoid species that in turn depress herbivore communities. Specialized enemies such as parasitoids use both visual and volatile cues from plant hosts and their prey items when hunting. In invaded habitats novel plants may initially mask prey presence, although novel cues can be learned after successful foraging (
Phylogenetic tools are rapidly being applied to the study of plant interactions, community dynamics, and invasion. Phylogenetic similarity between host plants can be associated with similarity in herbivory levels (
Diversity is an important response variable in ecology, a major ecosystem service, and sometimes a predictor of relative sensitivity to perturbation at larger scales. Plant invasions in general have been shown to reduce diversity of native plant species (
The interactions between arthropods and plants are complex and reciprocal. Plant invasions offer an interesting and unique opportunity to study these dynamics not only where arthropod-plant relationships have not developed, perhaps due to a lack of evolutionary history, but also in instances where new arthropod-host plant relationships have begun to emerge (
1 Integrate a biogeographic contrast of invasion with even a coarse but robust community arthropod sampling regime to comprehensively assess the mechanisms surrounding plant invasions. This might entail documenting at least the proportion of predators vs. prey items, and if possible the specific feeding mode of each arthropod (e.g. specialist or generalist), to clarify the direction and mechanism by which herbivore controls are acting on invasive plants similar to what has been detailed in food web studies (
2 Consider the role of plant functional group and by extension the complexity (or simplicity) of the native and exotic vegetation, and how this may mediate arthropod community interactions at all trophic levels (i.e. enemy-free space; resources available to arthropods). An extension of this concept could involve plant functional groups as they relate to plant primary and secondary defense compounds against herbivores, and the role this might play in trophic interactions.
3 Contrast the phylogenetic distances of invaders vs. native species where possible to elucidate mechanisms by which arthropod communities interact, both arthropod-arthropod and arthropod-plant. An invader that shares relatives (i.e. same family or genus) in a receiving community might be more amenable to hosting native arthropods by nature of similar morphology and chemistry than phylogenetically distinct invaders.
We thank N. Yan and R. Callaway for direction and helpful comments. The thorough and constructive feedback from two anonymous reviewers is highly appreciated. A Natural Sciences and Engineering Research Council of Canada’s Discovery Grant to CJL and Funding provided by York University to RDS supported this research. This is publication #72 of NSERC-CANPOLIN.
List of family-level phylogenies used to construct the master phylogeny. (doi: 10.3897/neobiota.16.4190.app1) File format: Micrisoft Word Document (docx).
Phylogenetic tree contrasting invasive and native plant species. (doi: 10.3897/neobiota.16.4190.app2) File format: Tree Files (tre).