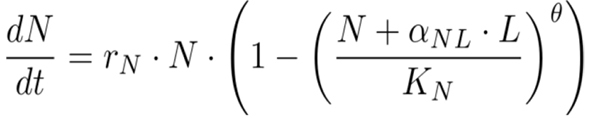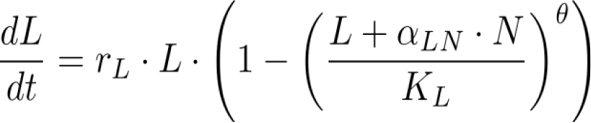(C) 2013 Meike J. Wittmann. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
The cladoceran Daphnia lumholtzi is a subtropical and tropical zooplankter, and an invasive species in North America. Thus far, Daphnia lumholtzi has not been detected in Europe. Here we investigated whether a hypothetical introduction to Europe could result in a successful invasion, either now or in the near future when facilitated by climate change. In laboratory experiments, we tested whether different clones of Daphnia lumholtzi can invade a resident community consisting of native Daphnia from lake Klostersee, Germany, and how invasion success depends on temperature and the presence or absence of planktivorous fish. In some treatments, invasion success was consistently high, and Daphnia lumholtzi reached densities similar to the native competitors by the end of the experiment. The presence of a planktivorous fish reduced the invasion success of Daphnia lumholtzi, and a clone with an inducible defense against fish predation was a more successful invader than a permanently defended clone. Of the three temperatures tested in this study (15, 20, and 24 °C), invasion success was highest at 20 °C. To understand the competitive interaction between native and introduced Daphnia, we fit a Lotka-Volterra-type competition model to the population dynamics. Our experimental and modeling results suggest that Daphnia lumholtzi can invade European lakes and can cause substantial declines in the population size of native Daphnia, with potential consequences for higher trophic levels.
invasive species, competition, climate change, inducible defenses
In their attempt to understand the determinants of invasion success, most studies focus on invasions that have already occurred. For example, many studies try to identify characteristic traits of invasive species, using data from previously successful invaders (
We follow such a mechanistic approach here, using the example of a possible invasion by the cladoceran Daphnia lumholtzi Sars in Europe. This zooplankter is native to subtropical and tropical regions of Africa, Australia and Asia, where it is found up to the Middle East (
A number of studies have investigated how the invasion success of Daphnia lumholtzi in North America depends on temperature, intensity of fish predation and competition with native North American zooplankton species. Since Daphnia lumholtzi experiences high temperatures across its native range, it is not surprising that it is well adapted to the high summer temperatures of water bodies in the south-central US. Indeed, when temperatures rise above 25 °C in late summer and the populations of native Daphnia species decline, Daphnia lumholtzi reaches its highest density (
Unique characteristics of Daphnia lumholtzi are the long head and tail spines that most clones form in response to chemical cues released by fish (
Temperature and predation do not only influence the growth rate of Daphnia lumholtzi, but also change the way this introduced species competes with native zooplankton species, such as native North American Daphnia.
In the popular statistical approaches used to predict future invasions (e.g. the ecological niche modeling approach introduced above), biotic interactions are usually neglected or assumed to be constant (
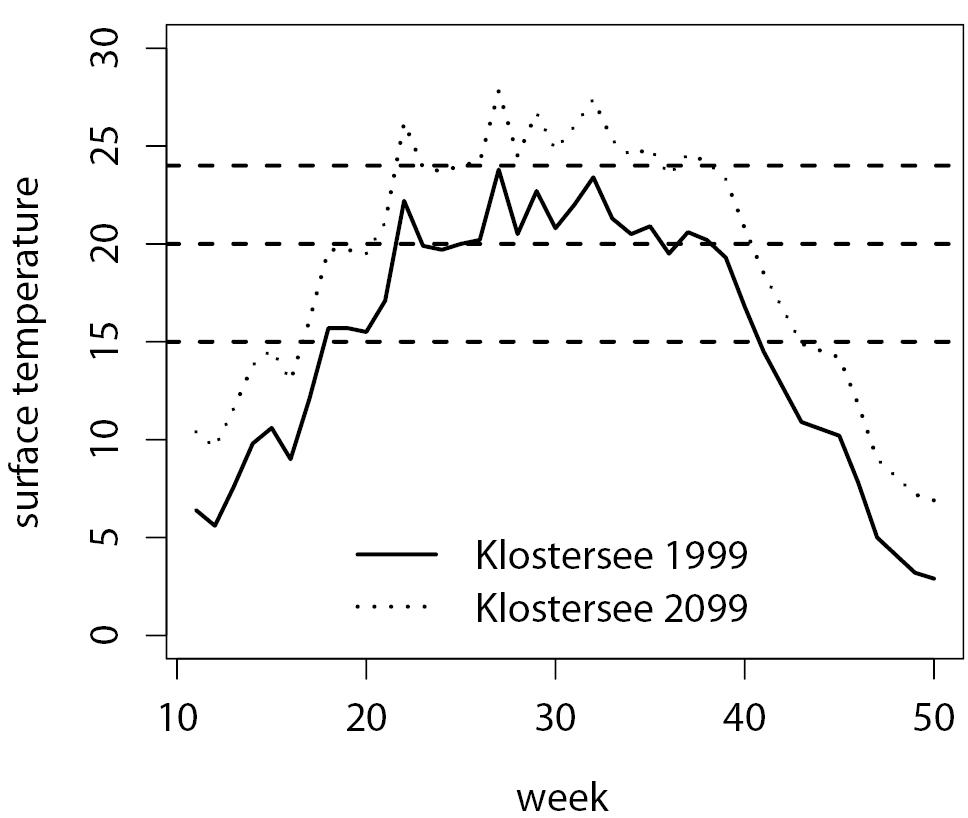
Observed surface temperature in Lake Klostersee in 1999 (solid line) and predicted surface temperature in 2099 under climate change scenario A1FI (dotted line, see
We collected native Daphnia in September 2009, using plankton nets in the middle of Lake Klostersee and performing several vertical hauls. Each clone used in our experiment consisted of the descendants of a single female from this original sample. Prior to the experiments, we kept the clones at 20 °C in semiartificial Daphnia medium based on ultrapure water, phosphate buffer and trace elements, and regularly fed them with Scenedesmus obliquus, a species of green algae which is commonly found in European lakes (see e.g.
The bitterling (Rhodeus amarus), a planktivorous fish native to Middle Europe, served as an experimental predator. The fish we used were approximately 4.5 cm long. This length compares to 2–3.5 cm for bluegill sunfish used by
The first experiment took place from June to July 2010. The eight treatments differed in temperature (20 or 24 °C), introduced clone (TE or AZ), and predation regime (predation by a bitterling for 10 min per day or no predation) in a fully factorial design. Each treatment was replicated five times resulting in a total of 40 experimental units. The experiment was carried out in 30-L white polypropylene containers with semiartificial Daphnia medium which were placed in climate chambers with a 12 h:12 h light:dark cycle. We added 0.5 mg C/L of green algae (Scenedesmus obliquus) to each unit every second day. Algae were cultured in artificial Z medium (
To create the resident native communities, we divided 60 L of a culture of each of the two native clones approximately equally into 40 portions, each with on the order of magnitude of 102 individuals. The portions were assigned randomly to the 40 experimental units and used to inoculate 15 L of Daphnia medium in each of them. One week after inoculation, we added 10 L of fresh medium to each unit, and one bitterling to each unit in the predation treatments. For most of the time, the fish were caged in a 5-L polypropylene container floating inside the experimental container. This served to avoid elimination of the entire population while guaranteeing a permanent release of predator-borne cues. The bottom and sides of this small container were removed and replaced by a 200-μm mesh, such that chemicals produced by the fish were exchanged through the mesh but Daphnia could not pass. The experimental units in the non-predation treatment had an empty 5-L container. Once a day, we released the fish for 10 min into the main compartment of the experimental unit and allowed them to feed on the Daphnia. After recapturing the fish, we provided them with dead defrosted red mosquito larvae (Chironomidae) as additional food.
Two weeks after inoculation (time 0), we introduced 25 Daphnia lumholtzi individuals (AZ or TE, depending on the treatment) into each experimental unit. Twenty-five is a number of individuals that we expected to be large enough to make chance extinctions unlikely but that was still small compared to the size of the native population at time 0. These founding individuals had been randomly sampled from populations grown at 20 °C. To maximize the contrast between the two clones, the AZ clone had been exposed to fish kairomones during the week prior to introduction, whereas the TE clone was naive to fish at the time of introduction. We sampled 10% of the volume before the introduction at time 0, and we sampled 5% of the volume every seven days until the end of the experiment. We filtered the sampled volume through a 125-μm mesh and preserved the Daphnia retained by the mesh in 70% ethanol. At the time of sampling, we randomly redistributed the fish in the predation treatments on the experimental units within one temperature treatment.
On day 1, four fish accidentally escaped from their containers (three units in the 24 °C, AZ treatment; one unit in the 24 °C, TE treatment), so that they were able to feed on the Daphnia of their experimental unit for an entire night. Since this lead to a strong decline in population densities, we decided to restock the respective units with approximately 500 native Daphnia (250 of each of the two clones) and six Daphnia lumholtzi. We determined this ratio by dividing the 25 introduced Daphnia lumholtzi individuals by the count of native Daphnia in the sample that we had taken 3 days before.
In the predation treatments, fish metabolic end products accumulated over time and apparently inhibited Daphnia population growth. Thus, after 21 and 28 days, we replaced one third of the volume in each unit with fresh medium. To remove the old medium, we used an aquarium pump covered by a 125-μm mesh such that no Daphnia were lost from the units during medium exchange. To avoid extinction of the entire Daphnia community, from day 21 onward the fish were only put into their small containers for one hour per day and were not allowed to feed on the Daphnia anymore.
The experiment ended on day 35. At this time, we sampled 1.25 L from the units in the non-predation treatment, whereas we examined the total volume in the predation treatment due to lower numbers of remaining individuals there. We counted the complete samples under a stereomicroscope at a magnification of 16. However, in predation units that contained more than 50 individuals in the previous week’s sample, we counted only 10% of the sample. Only individuals with clear contours of eye and body were counted, assuming that they were alive at the time of sampling. We distinguished native Daphnia and Daphnia lumholtzi according to the shape of their heads and tail spines.
The second invasion experiment took place from March to April 2011. To better understand the observations made in the first experiment, we changed the experimental design in several points. We now chose the temperature treatments 15 °C and 20 °C in order to cover a wider range of temperatures. Because we suspected that the white container walls in the first experiment made it easy for the fish to spot Daphnia, we used black containers in the second experiment. We hypothesize that the light conditions in these black containers are more similar to those in natural lake environments. Because the chemical conditions in the containers had deteriorated over the course of the first experiment, we decided to regularly exchange medium in the second experiment. However, large-scale medium exchange is logistically challenging, and thus we had to reduce the experimental volume to 10 L.
We inoculated native communities in 5 L medium and filled up the containers to 10 L six days later. For the first 11 days, fish were allowed to feed for only 5 minutes per day, later 10 minutes per day. To avoid the accumulation of fish chemicals, the fish were not permanently present in the experimental units, but only while feeding. For the rest of the day, we kept them together in an aquarium in 10 L of medium at the same temperature. Every day, we filtered the medium from the aquarium and used it to replace 1 L of medium from each unit in the predation treatment. In this manner, we simulated the permanent presence of fish in these units. In the other experimental units, we replaced 1 L by fresh medium every day. Among the 25 introduced Daphnia lumholtzi, 5 were embryo-bearing females whereas the other 20 were randomly selected from the population. The second invasion experiment ended on day 42. Two treatment combinations (TE clone without predation at 15 °C and 20 °C), however, were continued as a long-term experiment until day 91. During this additional time, we exchanged 7 L of medium once per week. We used the following light-dark cycle: 11.5 h light: 0.5 h dusk: 11.5 h night: 0.5 h dawn. All other parameters such as food supply were identical to the first experimental setup.
If Daphnia lumholtzi individuals were present in the final sample from an experimental unit, we say that Daphnia lumholtzi successfully established in this unit. To obtain a more quantitative measure for invasion success and the resulting change in community structure, we then analyzed the proportion of Daphnia lumholtzi at the end of the experiment. We modeled this response variable and its dependence on the treatment variables using logit-link binomial generalized linear mixed models as implemented in the package lme4 (
Li~Bin(Li+Ni, pi) (1)
and
logit(pi)=c1 + c2 · T(i)+ c3 · 1predation(i)+ c4 · 1TE(i)+ c5 · T(i) · 1predation(i)+
c6 · T(i) · 1TE(i)+ c7 · 1predation(i) · 1TE(i)+ c8 · T(i) · 1predation(i) · 1TE(i)+ ai (2),
where Li and Ni are the numbers of Daphnia lumholtzi and native Daphnia in the last sample in unit i, T(i) is the temperature in °C for unit i, 1predation(i) is 1 if unit i is in the predation treatment and 0 otherwise, 1TE(i) is 1 if the TE clone is introduced in unit i and 0 otherwise, and
ai~N(0, σa2) (3)
(see
To better understand the competitive dynamics in the long-term experiment, we fit a θ-logistic Lotka-Volterra competition model described by a system of ordinary differential equations
(4)
and
(5)
to the time series of population densities by minimizing the sum of the squared residuals with the L-BFGS-B method implemented in R’s optimization function. In this model, N is the native population size, L the population size of Daphnia lumholtzi, rN and rL are the respective intrinsic growth rates, KN and KL the carrying capacities, αNL and αLN the competition coefficients, and θ a parameter that determines the strength of density regulation.
The data underpinning the analyses reported in this paper are deposited in the Dryad Data Repository at doi: 10.5061/dryad.d5c67
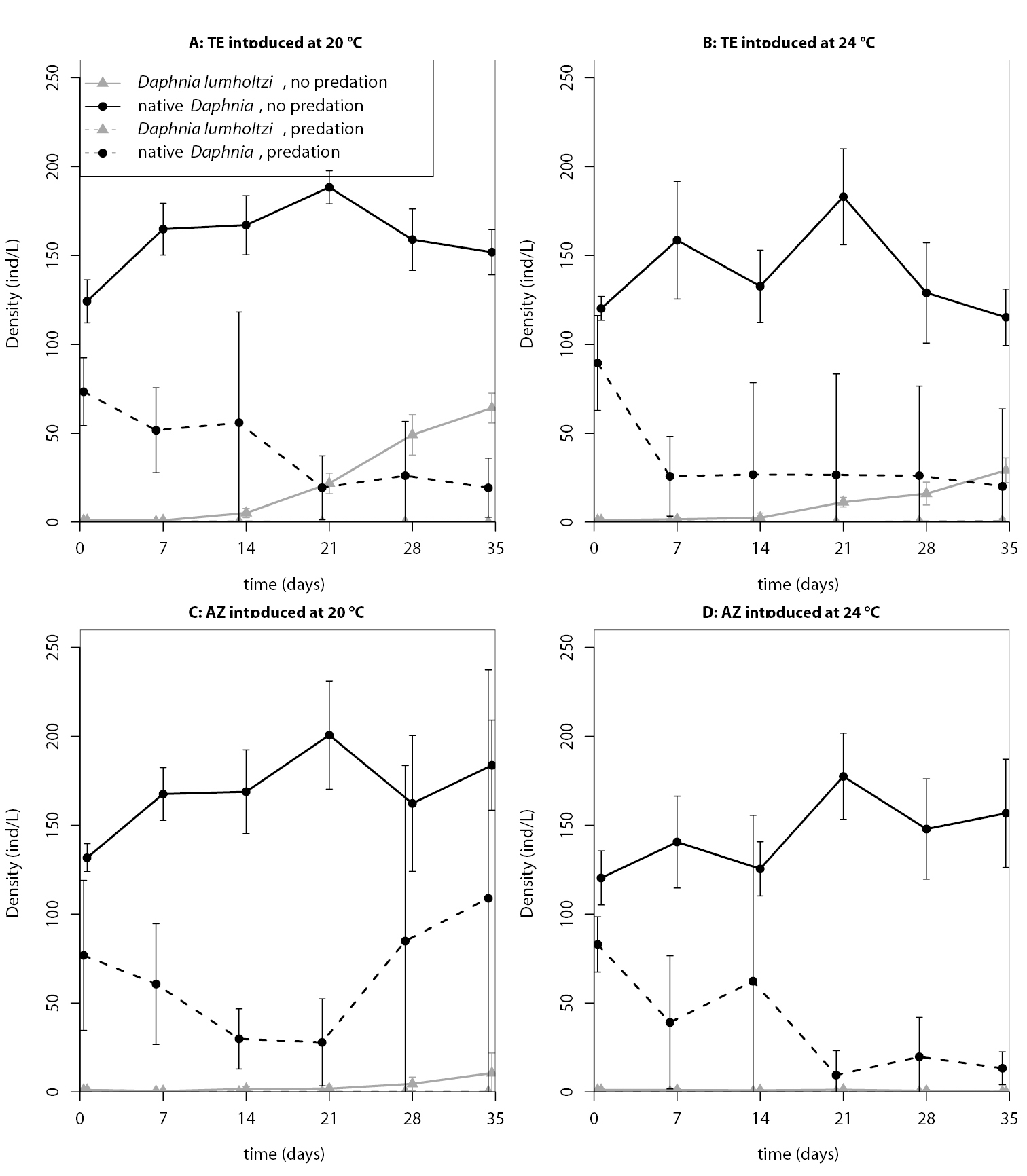
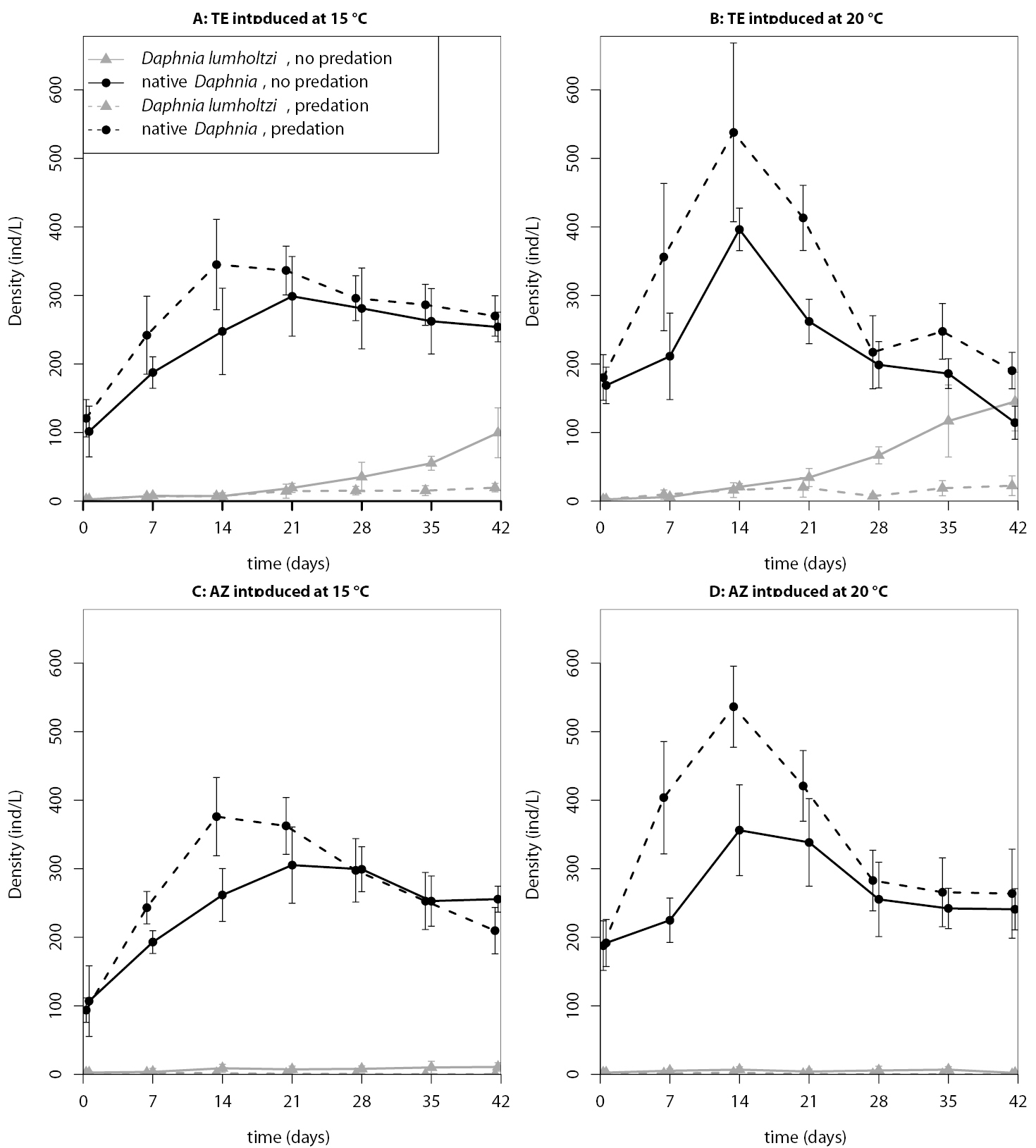
In both invasion experiments, the inducibly defended Texas clone established in all experimental units without predation (Tables 1 and 2). In the first invasion experiment, with one exception, all invasions in the predation treatment failed, whereas in the second experiment, the Texas clone established successfully under predation, and only the permanently defended Arizona clone failed consistently. In the absence of a predator, the Arizona clone had mixed establishment success. Temperature, predation, and the identity of the introduced clone, as well as the interactions between these factors, also strongly affected the population dynamics of introduced and native Daphnia (see Figs 2 and 3 for time-series plots). Consequently, the statistical models that best explained the proportion of Daphnia lumholtzi at the end of the experiments (lowest AIC score) included temperature, predation and clonal identity. The selected model for the second experiment also included all three two-way interactions but not the three-way interaction. On the other hand, for the first experiment, the interaction between predation and clonal identity was not part of the selected model (see Table 3 for estimated model coefficients and Table 4 for the AIC values of all candidate models). We used the estimated model coefficients to compute the final proportion of Daphnia lumholtzi that the models predict for the different treatment combinations (values in parentheses in Tables 1 and 2). Throughout, the Texas clone reached higher densities than the Arizona clone. Predation prevented, or at least slowed down, the population growth of Daphnia lumholtzi, especially that of the Arizona clone. In both experiments, the Texas clone at 20 °C had the highest invasion success.
Fraction of replicates with Daphnia lumholtzi establishment for the different treatments in the first invasion experiment, and the corresponding final proportion of Daphnia lumholtzi, as predicted by the selected generalized linear mixed-effects model (in parentheses).
| No predation | Predation | |||
|---|---|---|---|---|
| TE clone introduced | AZ clone introduced | TE clone introduced | AZ clone introduced | |
| 20 °C | 5/5 (0.296) | 5/5 (0.044) | 0/5 (<0.001) | 0/5 (<0.001) |
| 24 °C | 5/5 (0.199) | 0/5 (<0.001) | 1/5 (0.017) | 0/5 (<0.001) |
Fraction of replicates with Daphnia lumholtzi establishment for the different treatments in the second invasion experiment, and the corresponding final proportion of Daphnia lumholtzi, as predicted by the best generalized linear mixed-effects model (in parentheses).
| No predation | Predation | |||
|---|---|---|---|---|
| TE clone introduced | AZ clone introduced | TE clone introduced | AZ clone introduced | |
| 15 °C | 5/5 (0.274) | 5/5 (0.039) | 5/5 (0.066) | 0/5 (<0.001) |
| 20 °C | 5/5 (0.557) | 3/5 (0.008) | 5/5 (0.101) | 0/5 (<0.001) |
Time series of the population densities (means ± standard deviations) in the first invasion experiment.
Time series of the population densities (means ± standard deviations) in the second invasion experiment.
Estimated model coefficients (ci in equation 2) for the best generalized linear mixed-effects model for the proportion of Daphnia lumholtzi in the community.
| coefficient for experiment 1 | coefficient for experiment 2 | |
|---|---|---|
| intercept (c1) | 102.706 | 1.66 |
| temperature (c2) | -5.289 | -0.32 |
| 1predation (c3) | -128.150 | -17.21 |
| 1TE (c4) | -100.945 | -6.25 |
| temperature · 1predation (c5) | 5.230 | -0.15 |
| temperature · 1TE (c6) | 5.157 | 0.57 |
| 1predation · 1TE (c7) | 0 | 17.75 |
| temperature · 1predation · 1TE (c8) | 0 | 0 |
| σa | 0.46 | 0.28 |
Model selection for the proportion of Daphnia lumholtzi at the end of the experiment. The lowest AIC value for each experiment is highlighted in bold and indicates the respective selected model.T represents the effect of temperature, P predation, and C clonal identity; ai is a normally distributed random variable that is independently drawn for each experimental unit i.
| Model | AIC (experiment 1) | AIC (experiment 2) |
|---|---|---|
| T + P + C + T × P + T × C + P × C + T × P × C + ai | 63.13 | 71.63 |
| T + P + C + T × P + T × C + P × C + ai | 61.13 | 69.63 |
| T + P + C + T × P + T × C + ai | 59.13 | 75.24 |
| T + P + C + T × P + P × C + ai | 81.08 | 92.55 |
| T + P + C + T × C + P × C + ai | 77.29 | 71.89 |
| T + P + C + T × P + ai | 80.71 | 96.61 |
| T + P + C + T × C + ai | 77.34 | 75.97 |
| T + P + C + P × C + ai | 96.27 | 90.58 |
| T + P + C + ai | 94.83 | 94.62 |
| T + P + ai | 115.83 | 168.04 |
| T + C + ai | 131.02 | 134.34 |
| P + C + ai | 96.16 | 94.10 |
| T + ai | 139.681 | 174.83 |
| P + ai | 114.83 | 166.04 |
| C + ai | 129.90 | 132.35 |
| ai | 138.33 | 172.84 |
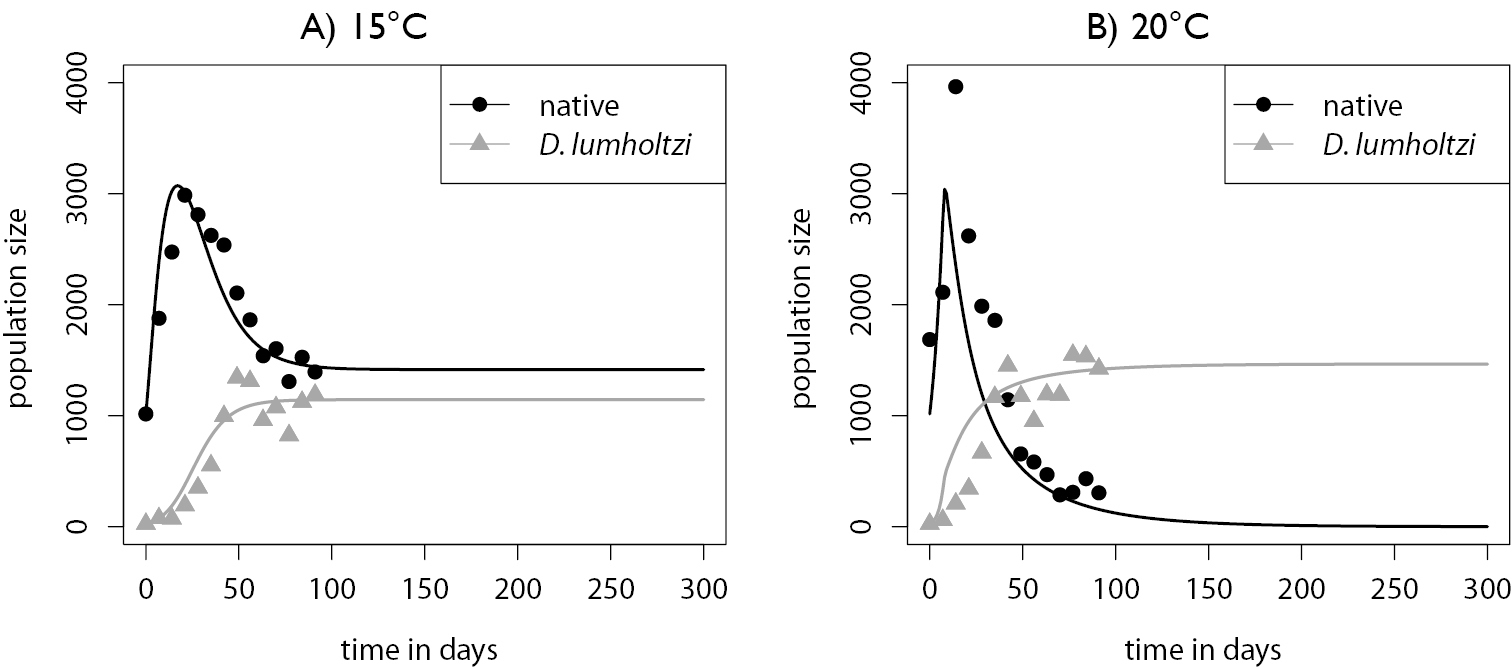
The long-term experiments provided additional insights into the influence of temperature on the invasion success of the Texas clone and its interaction with the native Daphnia. The simple Lotka-Volterra model (eqs. 4 and 5) together with the set of estimated parameters produces a satisfactory fit to the competitive dynamics (Fig. 4). At the cooler temperature of 15 °C, native Daphnia had the higher estimated growth rate. This was reversed at 20 °C. Competition was intensified with the increase in temperature and highly asymmetric at both temperatures, with Daphnia lumholtzi having much higher competitive effects on the native Daphnia than vice versa. The estimated carrying capacities for both species were higher at 20 °C than at 15 °C, and under both conditions, Daphnia lumholtzi had a smaller estimated carrying capacity than native Daphnia. The difference in the estimates for the parameter θ indicates that density dependence is stronger at 15 °C than at 20 °C. Furthermore, the Lotka-Volterra model allowed us to extrapolate the population dynamics and predict that at 20 °C, the native Daphnia would eventually reach a very low density or even go extinct, whereas at 15 °C coexistence with Daphnia lumholtzi would be possible.
Time series of native and introduced Daphnia in the long-term experiments with the fitted and extrapolated Lotka Volterra model.<br/> A) 15 °C with estimated model parameters: <br/> rN=0.412, rL=0.204, αNL=1.88, αLN=0.000, KN=3.57 · 103, KL=1.16 · 103, θ=0.505 with a residual sum of squares RSS =1242993<br/> B) 20 °C with estimated model parameters:<br/> rN=0.143, rL=0.369, αNL=3.120, αLN=0.308, KN=4.53 · 103, KL=1.47 · 103, θ=15.3 and RSS = 940080.
In our experimentally simulated introductions to European lakes, Daphnia lumholtzi had a high invasion success in the absence of predators. This was particularly true for the inducibly defended Texas clone. In most successful invasions, Daphnia lumholtzi reached high densities over the course of the experiment and substantially reduced the population size of native Daphnia.
Surprisingly and in contrast to the results of
Additional observations that we made indicated that the defense of Daphnia lumholtzi was effective against some of the fish in our experiments (see also Methods section above). However, the effect of the defense could have been counteracted by the fact that fish are visual, size-selective predators and therefore might have preferred Daphnia lumholtzi over native Daphnia: Daphnia lumholtzi are better visible than native Daphnia, due to their larger body size, their conspicuously colored broods, and their stronger tendency to produce ephippia. Selective predation for individuals with pigmented reproductive structures has been shown for other Daphnia species:
For the inducibly defended Texas clone, the weak performance in the predation treatment compared to the predator-free treatment could be partly due to the costs of developing the defenses. Following the classification of defense costs by
High costs of their large defense structures could also explain why the permanently defended Arizona clone was less successful than the inducibly defended Texas clone under all experimental conditions. These costs might be outweighed by the benefits only at a predation pressure higher than the one encountered in our experiment, an explanation that has also been suggested by
Temperature plays an important role for biological processes, from individual physiology to ecosystems. Therefore, climatic warming has the potential to affect biological invasions at all stages of the invasion process (
We must consider, however, that the establishment of a self-sustaining population of Daphnia lumholtzi in a European lake would also require populations to survive from year to year. Since Daphnia lumholtzi populations can persist in the form of resting eggs, it is not necessary for adults to be able to survive winter temperatures. Because resting eggs are produced sexually, and the encounter rate between mating partners can be reduced in small populations, Daphnia lumholtzi might be subject to an Allee effect (
At later invasion stages, changes in temperature may influence the growth and spread of established populations, for example by influencing their competitive abilities compared to native species (
Since we supplied only one algal species as resource, the potential species coexistence suggested by our modeling results is surprising at first sight. One possible explanation is intraspecific interference (
This is an example for the more general problem that our native Daphnia, fish, and algae represent only a small subset of the actual native community in a natural lake. In other areas of ecological research, a field study would be a good way to test hypotheses in a more realistic setting. However, in a study on potential future invasions, this is obviously too hazardous. A safer but challenging avenue of future research is to use more complex food webs in laboratory experiments. The differential success of the two Daphnia lumholtzi clones in our study highlights that it can even be important to include a set of genotypes within the same species. Such differences in invasion success between genotypes within one introduced species have also been reported by
Assuming that the detrimental effects of the presence of fish detected in our study do not, or less strongly, act in the field, our experiments did not identify any obstacles to an invasion of Daphnia lumholtzi in European lakes. A successful clone could be similar to the inducibly defended Texas clone, which can grow and compete for food at temperatures at least as low as 15 °C. Using our results and prior knowledge on the interaction of Daphnia lumholtzi with North American communities, what can we conclude about the potential impacts of Daphnia lumholtzi in the case of an invasion into European lakes? In contrast to some studies that found only weak effects of competition and suggest that Daphnia lumholtzi might be filling an empty niche in North America (
We would like to thank Mechthild Kredler for support in the laboratory, Achim Weigert for help with native Daphnia sampling, Sabine Gießler for identification of native Daphnia, Dirk Metzler for statistical advice, Katharina Engel for helpful discussions on Daphnia lumholtzi, as well as Jennifer Lohr, Wolfgang Rabitsch, and an anonymous reviewer for comments on the manuscript. JMJ acknowledges financial support from the Deutsche Forschungsgemeinschaft (DFG; JE 288/4-1).