(C) 2013 Kevin Jancowski. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Invasive alien American bullfrog populations are commonly identified as a pernicious influence on the survival of native species due to their adaptability, proliferation and consequent ecological impacts through competition and predation. However, it has been difficult to determine conclusively their destructive influence due to the fragmentary and geographically dispersed nature of the historical database. An expanding meta-population of invasive American bullfrogs, Rana catesbeiana (= Lithobates catesbeianus), becameestablished on southern Vancouver Island, British Columbia, Canada in the mid- to late 1980s. An on-going bullfrog control program begun in 2006 offered a unique opportunity to examine the stomach contents removed from 5, 075 adult and juvenile bullfrogs collected from 60 sites throughout the active season (April to October). Of 15 classes of organisms identified in the diet, insects were numerically dominant, particularly social wasps and odonates (damselflies and dragonflies). Seasonality and site-specific habitat characteristics influenced prey occurrence and abundance. Native vertebrates in the diet included fish, frogs, salamanders, snakes, lizards, turtles, birds, and mammals, including some of conservation concern. Certain predators of bullfrog tadpoles and juveniles are commonly preyed upon by adult bullfrogs, thereby suppressing their effectiveness as biological checks to bullfrog population growth. Prey species with anti-predator defences, such as wasps and sticklebacks, were sometimes eaten in abundance. Many prey species have some type of anti-predator defence, such as wasp stingers or stickleback spines, but there was no indication of conditioned avoidance to any of these. Results from this study reinforce the conclusion that, as an invasive alien, the American bullfrog is an opportunistic and seemingly unspecialized predator that has a uniquely large and complex ecological footprint both above and below the water surface.
Bullfrog, Rana catesbeiana, Lithobates catesbeianus, predation, diet, invasive species
The American bullfrog, Rana catesbeiana (= Lithobates catesbeianus), is widely considered one of the most ecologically destructive of invasive alien vertebrate species (
Stomach contents analyses from both native and invasive alien populations.
| Location | Invasive alien status | Sample size | Number of sites | Reference |
|---|---|---|---|---|
| Argentina: Buenos Aires | Non-native | 35 | 3 |
|
| Brazil: Minas Gerais | Non-native | 113 | 1 |
|
| Canada: British Columbia | Non-native | 13 | 1 |
|
| Canada: British Columbia | Non-native | 150 | 4 |
|
| China: Daishan Island | Non-native | 121 | 1 |
|
| Germany: Baden Wuerttemberg | Non-native | 44 | 1 |
|
| Japan: Kyoto | Non-native | 128 | 1 |
|
| USA: California | Non-native | 5 | 1 |
|
| USA: California | Non-native | 30 | 1 |
|
| USA: California | Non-native | 107 | 2 |
|
| USA: Michigan | Native | 166 | 2 |
|
| USA: Missouri | Native | 455 | 1 |
|
| USA: Missouri | Native | 4 | 1 |
|
| USA: Nebraska | Non-native | 1 | 1 | |
| USA: Nevada | Non-native | 28 | 2 |
|
| USA: New Mexico | Non-native | 138 | 1 |
|
| USA: New Mexico | Non-native | 85 | 1 |
|
| USA: Ohio | Native | 158 | 1 |
|
| USA: Ohio | Native | 1 | 1 |
|
| USA: Oklahoma | Native | 52 | 1 |
|
| Venezuela | Non-native | 338 | 1 |
|
| Total for all locations | 2172 | 29 |
From previous studies, a number of commonalities emerge. Bullfrogs consume a large number and variety of prey species (
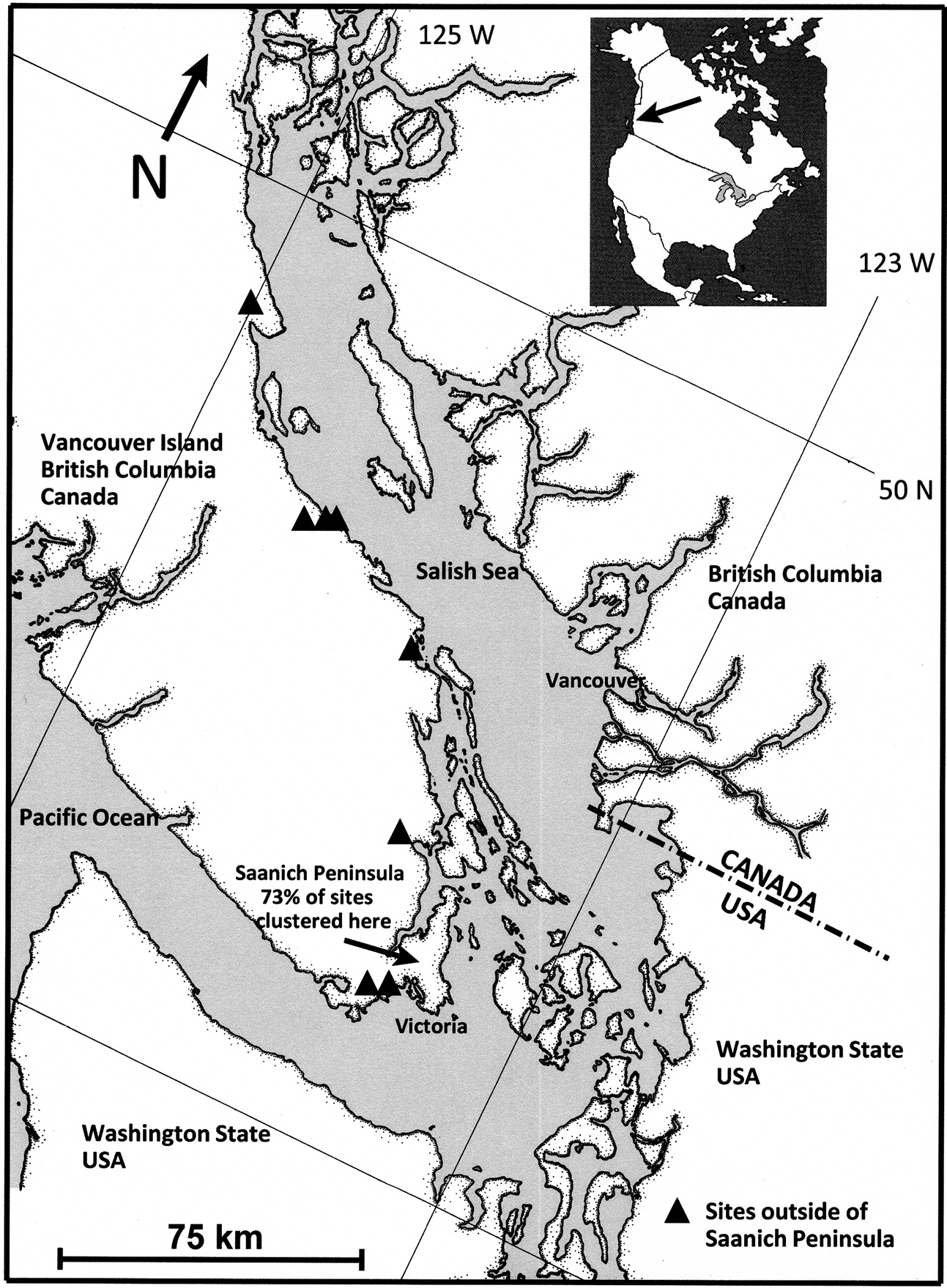
Populations of alien, invasive bullfrogs, geographically isolated and arising independently, are scattered along the southeast coast of Vancouver Island–their origins are often obscure. However, in the mid-1980s, a population of American bullfrogs became established just north of the City of Victoria at the extreme southern end of Vancouver Island (
The term “site” is used here, as in
Latitudinal range of study sites on southeastern Vancouver Island, British Columbia, Canada.
All fieldwork was carried out by one 2-person team working full-time, approximately 125 nights per season (April-September). Adult and juvenile bullfrogs were captured live using a patented manual “electro-frogger” technique that stuns them momentarily in the water so that they can be netted. They were later euthanized in a two-stage process that cooled them to torpor below 2⁰ C before being quick frozen (
A. Numbers of bullfrog stomachs with contents (91% of total examined), (B) without stomach contents (9% of total examined), and (C) with stomach contents as a percentage of monthly totals (with contents + without).
| A. | ||||||||
| Body length (mm) | April | May | June | July | August | September | Totals | % of Total |
| Juveniles < 80 | 338 | 496 | 212 | 224 | 397 | 453 | 2120 | 46 |
| Young males 80-120 | 70 | 113 | 182 | 214 | 313 | 102 | 994 | 22 |
| Mature males > 120 | 7 | 74 | 95 | 41 | 53 | 31 | 301 | 6 |
| Young females 80-120 | 110 | 111 | 111 | 139 | 242 | 212 | 925 | 20 |
| Mature females > 120 | 3 | 60 | 61 | 35 | 67 | 36 | 262 | 6 |
| Totals | 528 | 854 | 661 | 653 | 1072 | 834 | 4602 | 100 |
| % of Total with contents | 12 | 19 | 14 | 14 | 23 | 18 | 100 | |
| B. | ||||||||
| Body length (mm) | April | May | June | July | August | September | Totals | % of Total |
| Juveniles < 80 | 44 | 19 | 15 | 67 | 52 | 90 | 287 | 61 |
| Young males 80-120 | 8 | 9 | 8 | 7 | 3 | 3 | 38 | 8 |
| Mature males > 120 | 3 | 19 | 19 | 10 | 7 | 8 | 66 | 14 |
| Young females 80-120 | 14 | 4 | 2 | 5 | 6 | 10 | 41 | 8.5 |
| Mature females > 120 | 2 | 9 | 6 | 9 | 10 | 5 | 41 | 8.5 |
| Totals | 71 | 60 | 50 | 98 | 78 | 116 | 473 | 100 |
| % of Total | 15 | 13 | 11 | 21 | 16 | 24 | 100 | |
| C. | ||||||||
| April | May | June | July | August | September | Total Sample | ||
| Total sample (with contents + without) | 599 | 914 | 711 | 751 | 1150 | 950 | 5075 | |
| % with contents | 88% | 93% | 93% | 87% | 93% | 88% | 91% | |
Six calendar months were available for fieldwork (April to September, inclusive) but only one site was sampled in all six calendar months (Florence Lake, 48.4589, -123.5127). This site provided 33% (n = 1, 681) of the total sample. Conversely, 58% (n = 35) of the total sites sampled were each visited in only one calendar month of each of the 6 months available but these collectively produced only 10% (n = 516) of the total sample. Most of the total bullfrog sample (68%, n = 3, 455) came from 8 sites that were visited in at least 4 of the 6 months (Table S1, Table S2).
The range of organisms found in the stomachs of adult and juvenile bullfrogs spans 15 taxonomic classes (Table 3). The overall sample included 350 (7%) metamorphosed bullfrogs taken between 2006 and 2008; the entire 3, 835 caught in 2009 (76%); and 890 (17%) selected from a much larger sample from 2010. Contents from a total of 5, 075 bullfrog stomachs, collected over a five-year span, were ultimately examined (Tables 2A, 2B). Of all stomachs, 473 were found to be empty and were removed from the subsequent analyses of the remaining 4, 602 (Table 2A). A total of 18, 814 identifiable individual prey remains were recovered: 15, 081 (80%) of these from the 2009 series, 2, 612 (14%) from the 2010 series, and the remaining 1, 121 (6%) from 2006 to 2008.
Prey remains identified to class.
| Class | Total number of instances | % of total prey remains | % of bullfrog stomachs with contents | % of sites |
|---|---|---|---|---|
| Insecta | 15, 827 | 84.1 | 93.0 | 95 |
| Arachnida | 874 | 4.6 | 12.4 | 51 |
| Malacostraca | 770 | 4.1 | 10.9 | 50 |
| Gastropoda | 644 | 3.4 | 10.3 | 62 |
| Amphibia | 247 | 1.3 | 4.2 | 72 |
| Actinopterygii | 166 | 0.9 | 2.8 | 32 |
| Clitellata | 107 | 0.6 | 1.4 | 25 |
| Diplopoda | 59 | 0.3 | 0.9 | 20 |
| Mammalia | 40 | 0.2 | 0.9 | 32 |
| Aves | 25 | 0.1 | 0.6 | 27 |
| Chilopoda | 20 | 0.1 | 0.3 | 17 |
| Reptilia | 12 | 0.06 | 0.2 | 15 |
| Chelonia | 12 | 0.06 | 0.2 | 2 |
| Bivalvia | 8 | 0.04 | 0.1 | 5 |
| Gordioidea | 3 | 0.02 | 0.06 | 2 |
| Totals | 18, 814 | 100 |
Out of 18, 814 instances of identifiable remains, 84% were insects. Insects were also found in 93% of bullfrog stomachs and were consumed at 95% of the 60 sites sampled. The range in types of insects consumed is highly variable. Most insect parts were not identifiable to species but were at least attributable to one of 47 broader categories of variable taxonomic resolution (Table 4, Table S3).
Occurrence of individual prey remains identifiable as insect. The 21 most abundant insect prey categories are shown (See Table S3 for other insects identified).
| Insect group (adults unless specified) | Total # of instances | % of total prey items | % of bullfrog stomachs | % of sites | Seasonality: % cases / month | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Apr | May | June | July | Aug | Sept | |||||
| Social Wasp | 2, 674 | 14.0 | 16.0 | 50 | < 1 | < 1 | 1 | 13 | 64 | 22 |
| Aphid | 1, 982 | 10.0 | 4.9 | 20 | 1 | 2 | 1 | 2 | 71 | 24 |
| Damselfly | 1, 947 (17% nymph) | 10.0 | 23.0 | 68 | 2 | 18 | 35 | 13 | 25 | 7 |
| Dragonfly | 1, 415 (27% nymph) | 7.5 | 22.0 | 87 | 1 | 21 | 25 | 17 | 23 | 13 |
| Water Strider | 1, 259 | 6.7 | 12.0 | 41 | 42 | 13 | 17 | 12 | 11 | 5 |
| Unidentified Beetle | 1, 157 | 6.1 | 18.0 | 67 | 13 | 27 | 16 | 13 | 15 | 16 |
| Brachycera fly | 726 (61% larvae) | 3.8 | 8.9 | 42 | 4 | 3 | 7 | 10 | 21 | 55 |
| Ground Beetle | 675 | 3.6 | 9.6 | 67 | 20 | 26 | 15 | 7 | 19 | 13 |
| Nematocera fly (not crane flies) | 472 | 2.5 | 6.9 | 30 | 8 | 34 | 7 | 14 | 24 | 13 |
| Ant | 415 | 2.2 | 6.3 | 42 | 7 | 16 | 11 | 21 | 39 | 6 |
| Predaceous diving beetle | 399 | 2.1 | 6.8 | 67 | 12 | 31 | 18 | 9 | 23 | 7 |
| Butterfly/Moth | 365 (55% larvae) | 1.9 | 5.4 | 55 | 5 | 14 | 36 | 12 | 28 | 5 |
| Weevil | 324 | 1.7 | 4.6 | 28 | 6 | 12 | 4 | 13 | 18 | 47 |
| Other bee | 257 | 1.4 | 3.4 | 18 | 4 | 2 | 7 | 50 | 18 | 19 |
| Honey bee | 254 | 1.4 | 2.5 | 11 | 1 | < 1 | 8 | 70 | 16 | 5 |
| Unidentified insect | 234 | 1.2 | 4.6 | 47 | 13 | 19 | 16 | 11 | 20 | 21 |
| Back-swimmer | 225 | 1.2 | 3.4 | 50 | 2 | 30 | 25 | 9 | 8 | 26 |
| Caddisfly | 206 (10% larvae) | 1.1 | 2.8 | 28 | 38 | 45 | 6 | 1 | 5 | 5 |
| Non-social wasp | 124 | 0.7 | 2.4 | 31 | 3 | 6 | 13 | 22 | 41 | 15 |
| Click beetle | 108 | 0.6 | 2.0 | 27 | 23 | 52 | 19 | 3 | 3 | 0 |
| Giant water bug | 96 | 0.5 | 1.9 | 37 | 1 | 43 | 24 | 9 | 14 | 9 |
| Ladybird beetle | 87 | 0.5 | 1.6 | 18 | 3 | 5 | 3 | 12 | 33 | 44 |
At least 87% of adult and juvenile bullfrogs had food in their stomachs irrespective of month (Table 2C), although the species composition and densities of available prey change from month to month (Table 4). For example, dragonflies and damselflies were a dietary staple except in April, whereas social wasps were a dominant prey item but only in the late summer. Aphids were similarly important in the late summer but at only 20% of sites (Table 4). Late-summer prey also included brachyceran flies (particularly hoverfly larvae) (September), honey bees and other bees (July), and ladybird beetles (August-September) (Table 4). Water striders were especially significant at the start of the active season in mid-April (Table 4). They peaked in the diet of bullfrogs 60-70 mm in body length and then gradually dropped to zero in those over 140 mm. Giant water bugs were found in 27% of stomachs from one site (Filberg Marsh, May 27, 2010) but were relatively uncommon at most other sites.
Collectively, non-insect invertebrates made up just over 13% of prey remains with spiders and mites (Arachnida) at 4.6%, isopods and crayfish (Malacostraca) at 4.1%, and snails and slugs (Gastropoda) at 3.4% (Table 5). These three non-insect invertebrate classes all follow immediately behind Insecta (84%) in number of prey instances (Table 3). Gastropods had been eaten at 62% of sites, followed by Arachnida (52%), and Malacostraca (50%) (Table 3).
Non-insect invertebrate prey remains.
| Non-insect invertebrate group | Total # of cases | % of total prey remains | % of bullfrog stomachs | % of sites | Seasonality: % of cases / month | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Apr | May | June | July | Aug | Sept | |||||
| Spiders | 873 | 4.6 | 8.9 | 52 | 7 | 24 | 24 | 25 | 10 | 10 |
| Snails | 533 | 2.8 | 8.1 | 58 | 12 | 12 | 12 | 11 | 15 | 20 |
| Isopods | 481 | 2.6 | 5.3 | 40 | 22 | 17 | 17 | 6 | 9 | 26 |
| Crayfish | 174 | 0.92 | 2.8 | 22 | 2 | 17 | 17 | 18 | 52 | 6 |
| Amphipods | 115 | 0.61 | 0.24 | 2 | 0 | 62 | 62 | 9 | 1 | 0 |
| Slugs | 108 | 0.57 | 1.60 | 38 | 22 | 10 | 10 | 20 | 23 | 3 |
| Earthworms | 83 | 0.44 | 0.37 | 12 | 76 | 0 | 0 | 0 | 2 | 2 |
| Millipedes | 59 | 0.31 | 0.91 | 20 | 22 | 5 | 5 | 5 | 10 | 12 |
| Leeches | 24 | 0.13 | 0.48 | 20 | 17 | 8 | 8 | 0 | 13 | 37 |
| Centipedes | 20 | 0.11 | 0.33 | 17 | 0 | 33 | 33 | 20 | 7 | 7 |
| Clams | 8 | 0.04 | 0.11 | 5 | 0 | 25 | 25 | 0 | 0 | 12 |
| Mites | 1 | 0.01 | 0.02 | 2 | 0 | 0 | 0 | 100 | 0 | 0 |
Spiders (Arachnida) were the most frequently encountered non-insect invertebrate group (Table 5) but still ranked seventh overall behind the six dominant categories of insect. Unlike the seasonal and transient nature of many of the insect groups, spiders remained common prey throughout the active season (Table 5). After spiders, the next arthropod groups were isopods, in eleventh place overall, and crayfish (Malacostraca) in twenty-second. Crayfish figured in the diet at only 22%, of sites and their importance varied from site to site. For example, at one site they were found in 62% of stomachs, but these were taken from a relatively small series of only 16 bullfrogs. Aquatic snails ranked tenth in overall frequency while terrestrial slugs were in twenty-fifth place and found in 1.6% of bullfrog stomachs (Table 5).
Fish (Actinopterygii) and amphibians (Amphibia) were the dominant vertebrate prey, occurring in 2.8% and 4.2% of the stomachs, respectively (Table 3). Three-spined stickleback fish (Gasterosteus aculeatus) was the most common vertebrate prey species, but found in only 1.5% of bullfrogs stomachs and at just 27% of sites (Table 6). Their frequency in the diet varied from place to place, but at one site they were found in 26% of stomachs.
The top 14 vertebrate prey categories in the bullfrog diet (See Table S4 for other vertebrates identified).
| Vertebrate Group or Species | Total # of cases | % of total prey remains | % of bullfrog stomachs | % of sites | Seasonality: % instances/month | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Apr | May | June | July | Aug | Sept | |||||
| Three-spined stickleback (Gasterosteus aculeatus) | 97 | 0.52 | 1.5 | 27 | 3 | 30 | 19 | 11 | 6 | 31 |
| Pacific treefrog (Hyla regilla)–including tadpoles | 74 | 0.39 | 1.2 | 33 | 12 | 39 | 19 | 25 | 4 | 1 |
| Bullfrog juveniles (Rana catesbeiana) | 51 | 0.27 | 0.96 | 33 | 2 | 6 | 2 | 10 | 10 | 70 |
| Rough-skinned newt (Tarchica granulosa) | 50 | 0.26 | 0.87 | 21 | 0 | 36 | 18 | 8 | 26 | 12 |
| Bullfrog tadpoles | 30 | 0.16 | 0.43 | 15 | 0 | 7 | 33 | 30 | 27 | 3 |
| Sculpin (Cottus sp.) | 25 | 0.13 | 0.46 | 3 | 20 | 8 | 8 | 0 | 40 | 24 |
| Shrew (Sorex sp.) | 24 | 0.13 | 0.48 | 18 | 4 | 17 | 17 | 4 | 54 | 4 |
| Unidentified fish | 18 | 0.10 | 0.39 | 6 | 28 | 11 | 6 | 22 | 22 | 11 |
| Townsend’s vole (Microtus townsendi) | 16 | 0.08 | 0.35 | 13 | 0 | 25 | 31 | 0 | 25 | 19 |
| Pumpkinseed sunfish (Lepomis gibbosus) | 14 | 0.07 | 0.30 | 18 | 0 | 21 | 29 | 14 | 29 | 7 |
| Western painted turtle (Chrysemys picta) | 12 | 0.06 | 0.17 | 2 | 81 | 19 | 0 | 0 | 0 | 0 |
| Red-legged frog (Rana aurora) | 10 | 0.05 | 0.21 | 9 | 0 | 10 | 10 | 20 | 60 | 0 |
| Northwestern salamander (Ambystoma gracile) | 10 | 0.05 | 0.20 | 5 | 0 | 60 | 40 | 0 | 0 | 0 |
| Coho salmon (Oncorhynchus kisutch) | 9 | 0.05 | 0.13 | 2 | 0 | 0 | 0 | 100 | 0 | 0 |
Cannibalism of bullfrog juveniles and tadpoles collectively made up only 0.43% of total prey remains (Table 6). In one extraordinary instance, they were found in 48% of bullfrog stomachs from a single site. However, when all other records of amphibian predation [Pacific treefrogs, red-legged frogs, rough-skinned newts, ambystomatid salamanders (2 species), and plethodontid salamanders (2 species)] are combined (n = 159), they amount to almost exactly twice the number of instances of bullfrog cannibalism (n = 81) (Table 6, Table S4). Individual bullfrog stomachs were found to contain as many as 4 adult Pacific treefrogs and 3 adult rough-skinned newts. At one location, treefrogs were in the stomachs of 31% of bullfrogs sampled.
The majority (60%) of the 40 individual mammals consumed were shrews, while the rest were all Townsend’s voles (Table 6). There were eight passerine bird species represented by 25 records from 27% of the sites (Table S4). Of reptiles, three species of garter snakes (11 total snakes) were found in the diet along with a single northern alligator lizard (Table S4). Of special conservation concern were the 12 western painted turtle hatchlings (Class Chelonia) that equaled all reptile species combined as a percentage of total bullfrog prey (0.06%, Table 6, Table S4).
The approach used here is to focus primarily on instances of predation rather than on ingested volume or nutritional quality in the diet. We accept that one vertebrate is the nutritional equivalent of many insects or other invertebrates, but quantifying and analyzing the relative nutritional significance of each prey instance was beyond the scope of this study.
Insects were found in 93% of the 4, 602 bullfrog stomachs with contents, which is consistent with
This study found that early in the bullfrog active season, Odonata (dragonflies and damselflies; May: 45% adults; June: 81% adults) were a consistently important prey for all size-classes of bullfrogs, and this has also been reported by
Bullfrogs are seemingly immune to many natural predator defenses. Previous studies have alluded to the toxic or potentially repellent effects of natural prey defense mechanisms on predatory bullfrogs. For example,
Sticklebacks were the most numerous vertebrate prey and were also one of the most defensively armed. Bullfrogs, however, were seemingly immune to the discomfort of stickleback spines, and we recovered as many as five of these fish from a single stomach. Bullfrogs are reported to have eaten both scorpions and rattlesnakes along the lower Colorado River (
Dragonfly nymphs are known to prey on bullfrog tadpoles (
In 2011, we observed an adult common garter snake (Thamnophis sirtalis) eating a juvenile bullfrog, and this aquatic-foraging snake when at full adult size should be easily able to eat at least half-grown bullfrogs.
A giant water bug (Belostomatidae) was observed killing a bullfrog tadpole in captivity (K. Jancowski, personal observation), and they are known predators of other anurans including ranids (
Another organism found in the adult bullfrog diet, and also a predator of bullfrogs (
Bullfrogs routinely leave the water and migrate overland as adults and juveniles, presumably feeding as they travel. This may account for species turning up in the bullfrog diet that are strictly terrestrial, e.g. Townsend’s voles, terrestrial shrews, northern alligator lizards, western red-backed salamanders (Plethodon vehiculum), and Oregon ensatina salamanders (Ensatina eschscholtzii).
Aphids, because they are tiny, would seem to be an unlikely temptation to bullfrogs. However, aphids ranked second only to social wasps in number of instances of insect predation (Table 4). One probable explanation for this is that in late-summer aphids aggregate in large numbers to feed on the floating leaves of the yellow pond-lily (Nuphar polysepalum). The aphids, in turn, attract the attention of predatory wasps, dragonflies, damselflies, brachyceran flies, lacewings, and ladybird beetles, which also attract the interest of predatory bullfrogs. In the process of catching or attempting to catch these larger insects, bullfrogs are inadvertently picking up aphids on their sticky tongues. Approximately 55% of bullfrogs containing aphids had also eaten one or more of these associated species. Consequently, pond-lily leaves can be important feeding stations for bullfrogs as aphids gather on them in late summer.
Cannibalism, though well known to occur in bullfrogs, has not been very comprehensively studied (
We sampled 448 bullfrogs that were greater than or equal to 130 mm in body length, or comparable in body size to the “large” category in
In the absence of alternative prey, cannibalism remains an option for this species that would be of variable importance from site to site, season to season, and year to year. In the long-term, unmanaged bullfrog populations might conceivably drive down native species numbers to the point where cannibalism becomes increasingly important to bullfrog population sustainability.
Of native amphibians, the Pacific treefrog was the most frequently eaten by bullfrogs (Table 6). Treefrogs peaked in the bullfrog diet in May (39%) as male treefrogs migrate into the water to set up a mating chorus closely followed by females. At least 30% of treefrogs eaten in April and May were females, and 53% of these were gravid. Although bullfrogs are eating more adult males than adult females during this spawning period, the numerical loss of eggs to persistent (April to July) bullfrog predation could be substantial. Male treefrogs are likely in the water for a much longer interval than the females and they are making themselves more conspicuous by vocalizing (
Second to treefrogs are rough-skinned newts. Predation on newts peaked in May (36%) and then rose again in August (26%) (Table 6). These peaks coincide with the May migration of gravid adult female newts to the water to reproduce and the late-summer transformation of larval newts into terrestrial juveniles migrating away from the water (
Included in this study were the four sites sampled over 5 years by
The bullfrogs that figured in this study were not collected primarily for the purpose of examining their stomach contents. They were captured and euthanized as part of a research and development program exploring the feasibility and practicality of bullfrog eradication. This was carried out while testing and refining the electro-frogger technique on a regional scale. Most of the 60 sites included in this study (86%) were only visited in three or less of the six months available within the bullfrog’s active season, resulting in only 32% of the overall sample (Table S2). This is because, for the most part, they were smaller ponds where all of the adult and juvenile bullfrogs present could be removed in one or two evenings. In addition, there were a few single-evening reconnaissance missions to sites of interest. The remaining 14% of sites were the larger and more difficult ones where bullfrog densities, immigration rates, and problematical habitats required more effort to bring bullfrog numbers down. The most demanding (Florence Lake, 48.4589, -123.5127) was the only site visited in each of all six months (April to September) and produced 33% of the overall sample (Table S1, Table S2). Consequently, stomach contents from most sites are snapshots of what bullfrogs were eating at that particular site on a specific evening or over a few nights. The database compiled for this analysis is, therefore, a blend of a few sites sampled many times throughout the summer coupled with many sites visited only a few times each in a much more restricted time-frame. The regional database is comprehensive in terms of including samples collected nightly during the entire field season, but is fragmentary in terms of providing seasonally comparative datasets for most of the sites.
American bullfrogs have been identified as, or are suspected to be, a threat to the survival of various vertebrates world-wide, including native fish (
In British Columbia, three species of conservation concern relate to this study: the red-listed western painted turtle (Chrysemys picta bellii), the blue-listed northern red-legged frog (Rana (Lithobates) aurora), and, the aquatic-foraging and red-listed, American water shrew (Sorex palustris brooksi). Bullfrogs were found to be consuming hatchling western painted turtles as they entered the water. This was clear from the average carapace length of only 3 cm and the timing of these instances in late April and early May (Table 6). Any loss of hatchling painted turtles is a serious threat to turtle survival because the females produce few eggs and survivorship to recruitment is low (
It is of economic interest that coho salmon (Oncorhynchus kisutch) juveniles were found in 16% of bullfrogs sampled from Prior Lake in early July (Table 6, Table S1). Most of these were about 8 cm long, though bullfrogs have been documented eating trout up to 15 cm in length (
An organism that lies dormant for almost six months of the year must replenish its fat reserves during the relatively brief six-month active season. Mature adults, in particular, should have the life experience to be proficient hunters. They also have energy demanding roles that include vocalizations, territorial defense, egg production, spawning, and may include overland migrations. Then they must end the season with sufficient reserves to overwinter for another six months. The percentage of mature adults of both genders with empty stomachs was, therefore, remarkably high (Table 2B).
1 As an “invasive alien” the American bullfrog is a highly adaptable, opportunistic, and seemingly unspecialized predator that has a uniquely large and complex ecological footprint both above and below the water surface.
2 Insects were the dominate prey group found in 84% of prey instances and 93% of stomachs with food, but seasonality influenced the relative importance of any one insect group over another at any given time period.
3 Cannibalism was found to be a minor component of the diet in terms of relative instances and accounted for approximately 34% of all instances of predation on amphibians.
4 Bullfrog control measures should be routinely factored into management plans for rare and endangered species, such as the western painted turtle on southern Vancouver Island, which are particularly vulnerable to bullfrog predation.
This program was made possible thanks to the consistent funding of Victoria’s Capital Regional District (CRD) departments of Water Services and Parks & Community Services. Funding has also been gratefully received from various municipalities throughout the region including: Langford, Saanich, Highlands, View Royal, Metchosin, and Sooke, and agencies such as the Hartland Landfill and the Swan Lake-Christmas Hill Nature Sanctuary. Many private citizens also made financial contributions to the program. The Veins of Life Watershed Society and the Highlands Stewardship Foundation were instrumental in getting this program off the ground. Finally, we must thank Mr. David Nagorsen for mammal identification, Dr. Alex Peden for equipment and the base map adapted for this report, and Dr. Sylwia Jancowski for laboratory assistance.
Supplementary tables. (doi: 10.3897/neobiota.16.3806.app) File format: Microsoft Excel Document (xls).
Explanation note: Table S1: Sites where bullfrogs were collected on Vancouver Island, British Columbia, Canada. Table S2: Sampling frequency by month per site and its relation to catch. Table S3: Occurrence of individual prey remains identifiable as insect. The remaining 26 insect prey categories not given in Table 4. Table S4: The remaining 19 vertebrate prey groups in the bullfrog diet not shown in Table 6.