(C) 2013 Laura A. Sanderson. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Dog-strangling vine (Vincetoxicum rossicum) is an exotic plant originating from Central and Eastern Europe that is becoming increasingly invasive in southern Ontario, Canada. Once established, it successfully displaces local native plant species but mechanisms behind this plant’s high competitive ability are not fully understood. It is unknown whether cooler temperatures will limit the range expansion of Vincetoxicum rossicum, which has demonstrated high tolerance for other environmental variables such as light and soil moisture. Furthermore, if Vincetoxicum rossicum can establish outside its current climatic limit it is unknown whether competition with native species can significantly contribute to reduce fitness and slow down invasion. We conducted an experiment to test the potential of Vincetoxicum rossicum to spread into northern areas of Ontario using a set of growth chambers to simulate southern and northern Ontario climatic temperature regimes. We also tested plant-plant competition by growing Vincetoxicum rossicum in pots with a highly abundant native species, Solidago canadensis, and comparing growth responses to plants grown alone. We found that the fitness of Vincetoxicum rossicum was not affected by the cooler climate despite a delay in reproductive phenology. Growing Vincetoxicum rossicum with Solidago canadensis caused a significant reduction in seedpod biomass of Vincetoxicum rossicum. However, we did not detect a temperature x competition interaction in spite of evidence for adaptation of Solidago canadensis to cooler temperature conditions. We conclude that the spread of Vincetoxicum rossicum north within the tested range is unlikely to be limited by climatic temperature but competition with an abundant native species may contribute to slow it down.
Dog-Strangling Vine, Vincetoxicum rossicum, invasive species, invasion ecology, competition, phenotypic plasticity, climatic temperature range, spread, reproductive phenology
Dog-strangling vine (Vincetoxicum rossicum (Kleopow) Barbar.; syn. Cynanchum rossicum (Kleopow) Borhidi) is an alien invasive plant species from the Ukraine and south-western Russia that has established in the north-eastern United States and southern Ontario. Vincetoxicum rossicum was first found in Toronto in 1889 (
Currently, the distribution range of Vincetoxicum rossicum in North America has temperature and precipitation limits similar to those found in its native range (
It is unknown the extent to which climate serves as a barrier for further spread in the introduced range. Areas of central/northern Ontario that have, on average, slightly cooler temperatures than those in the vine’s current range might be at risk of invasion by this plant. As such, determining the phenology and fitness of Vincetoxicum rossicum under those conditions is required for risk assessment and to potentially adjust management practices.
Plant phenology can be influenced by abiotic and biotic factors (
Field studies on plant phenology have indicated that temperature cues have a large influence on flowering in many species (
On the biotic side, interspecific competition may be a limiting factor in species distribution (
The purpose of this study was two-fold. First, we investigated whether Vincetoxicum rossicum could grow under the cooler climate of northern Ontario, and whether any observed phenological changes could represent a barrier to the successful establishment of this invasive plant. Secondly, we tested the response of Vincetoxicum rossicum to the presence of a perennial abundant native plant (i.e., Canada goldenrod - Solidago canadensis L. var. canadensis) whose center distribution range is northern Ontario, and how the competitive interaction was affected by the shift in temperature. We selected Solidago canadensis as our competing species because it co-occurs with Vincetoxicum rossicum (
Dog-strangling vine (Vincetoxicum rossicum (Kleopow) Barbar.) root crowns were collected in Rouge Park, Toronto, ON (43.80526°N, 79.13594°W) in early May, 2011, before the onset of the growing season. The substrate used in the experiment consisted of soil collected from an un-invaded site adjacent to one invaded by Vincetoxicum rossicum. Root crowns were planted in one side of 10 L pots that were divided in half by a nylon mesh (30 μm opening) (Sefar Nitex 03-30/18, Heiden, Switzerland), which allows water and microbes, including fungal hyphae, to cross but prevents roots. The use of this nylon mesh still allows plants to compete for water and nutrients through diffusion, mass flow and mycorrhizal networks while preventing the roots from intertwining. The pots were filled with a 13 cm layer of a 2:1 mixture of Turface (a montmorillonite clay, Turface Athletics MVP, Profile Products LLC, Buffalo Grove, IL, USA) and non-calcareous granitic sand (Hutcheson Sand and Mixes, Huntsville, ON) followed by a 10 cm layer of 1.2 kg of field soil, and an additional 3 cm of the Turface:sand mixture.
The experiment consisted of a completely randomized block design with two crossed factors; ‘plant competition’ and ‘temperature’. Specifically, for ‘plant competition’ Vincetoxicum rossicum plants were either planted alone (control) or with a Canada Goldenrod (Solidago canadensis L. var. Canadensis; seeds were collected in the north eastern United States by Ontario Seed Company, Waterloo, ON, Canada) seedling in the other half of the pot (competition group). Solidago canadensis seedlings were also planted alone (Solidago canadensis control). Each of these treatments comprised a total of 24 pots, which were divided evenly among six reach-in controlled environment units (Conviron, Winnipeg, MN, Canada), each representing a block; three chambers were set to Toronto (TO) growing season temperatures and three chambers set to Sault Ste. Marie (SSM) growing season temperatures for an overall total of 72 pots. To minimize any potential variability among controlled environment units all respective pots were rotated among units and re-randomized within each block every 25 days. Toronto temperatures are on average approximately 3° C warmer than SSM. We used weather records collected by Environment Canada from 1980–2010 to simulate the monthly temperature conditions throughout the growing season (Table 1). We also used these data to simulate photoperiod throughout the growing season. Since photoperiod was similar between the two locations and the main goal was to test temperature effects, it was kept the same across treatments (Table 1).
Temperature regimes used to simulate Toronto (TO) and Sault Ste. Marie (SSM) growing seasons in the controlled environment units.
| Location | Range† | May | June | July | August | September |
|---|---|---|---|---|---|---|
| Simulated temperature (°C)∆ | ||||||
| TO | High | 20.3 | 25.5 | 28.4 | 27.4 | 23.0 |
| Average | 14.5 | 19.9 | 22.7 | 21.9 | 17.5 | |
| Low | 8.8 | 14.2 | 17.1 | 16.5 | 12.1 | |
| SSM | High | 17.9 | 22.8 | 25.5 | 24.8 | 20.3 |
| Average | 11.4 | 16.2 | 19.2 | 18.9 | 14.8 | |
| Low | 5.0 | 9.7 | 12.9 | 13.0 | 9.4 | |
| Simulated photoperiod (hours of light per day)‡ | ||||||
| 14h; 15h | 15h | 15h; 14h | 14h; 13h | 13h | ||
† Simulated temperatures were 1.4 °C warmer than the calculated average to meet the minimum range allowed by the spell out controlled environment units (i.e., 5.0°C).
∆ Maximum and minimum temperatures were each maintained for 6 hours, with the remaining 12 hours set at average temperatures.
‡ Average light intensity ranged between 350–400 μmol m-2 s-1. When two values are given, this indicates the changing day length during that month.
Plants were allowed to grow for five months (simulated “May” to “September”). During this time we recorded daily reproductive phenology measurements for Vincetoxicum rossicum (presence of first flower bud, first flower opening and seedpod production). Plants were watered to field capacity every second day to ensure that water deficiency was not a factor in the experiment. Since we noticed mild signs of nutrient deficiency in all plants, all pots were fertilized with Miracle-Gro 24:8:16 (The Scotts Company LLC, Mississauga, ON, Canada) (0.84 ppm P per pot) after the first month of growth. In addition all pots received a solution of 12-0-44 fertilizer (6 ppm N per pot) and slow-release 18-6-8, 70-day fertilizer (meaning that after 70 days, 80% of the nutrients would have been released into the soil; 0.72 ppm N per pot) (Nutricote, Plant Products Co., Brampton, ON, Canada) after the fourth month of growth. The use of low fertilizer concentrations and of a slow release fertilizer ensured that plants had sufficient nutrients to survive but that soil fertility was such that they had to compete for nutrients. In the final month of the experiment, plants experienced an outbreak of thrips in all chambers, and were sprayed with Nemasys nematode spray (50 million count, Becker Underwood, SK, Canada).
The commercial seed stock of Solidago canadensis was contaminated with other species of goldenrod and asters. This resulted in twelve pots (three alone and six in competition under TO temperatures and three in competition under SSM temperatures) containing the “wrong” plant species, which could not be differentiated until two months into the experiment. These pots have been removed from the competition data analysis, but have been kept for the Vincetoxicum rossicum phenology analysis.
At the end of the experimental period (as determined by simulated ‘first frost date’ for SSM), all plants were harvested. Roots and shoots were placed in separate bags, dried for three days at 60°C and weighed. Competitive response was calculated for both plant species according to methodology by
We analysed plant responses to temperature on phenological data (i.e., flower budding, open flowering and Seedpod formation), total plant biomass, root : shoot ratio, and competitive response. Since pots were rotated and re-arranged randomly among controlled environment units as blocks to minimize the potential for chamber effects, block effects were tested first within each temperature group using one-way ANOVAs. If a block effect was not detected, factorial ANOVAs were carried out. When testing phenology, total biomass, and root: shoot ratio each plant species was tested individually with the factor ‘plant competition’ being comprised of two levels (i.e., either Solidago canadensis or Vincetoxicum rossicum alone or the response of that species in presence of the competitor). To test for competitive response the model included ‘plant competition’ and ‘temperature’ as factors. Data were Box-Cox transformed to meet the test’s assumptions. All statistical analyses were carried out using
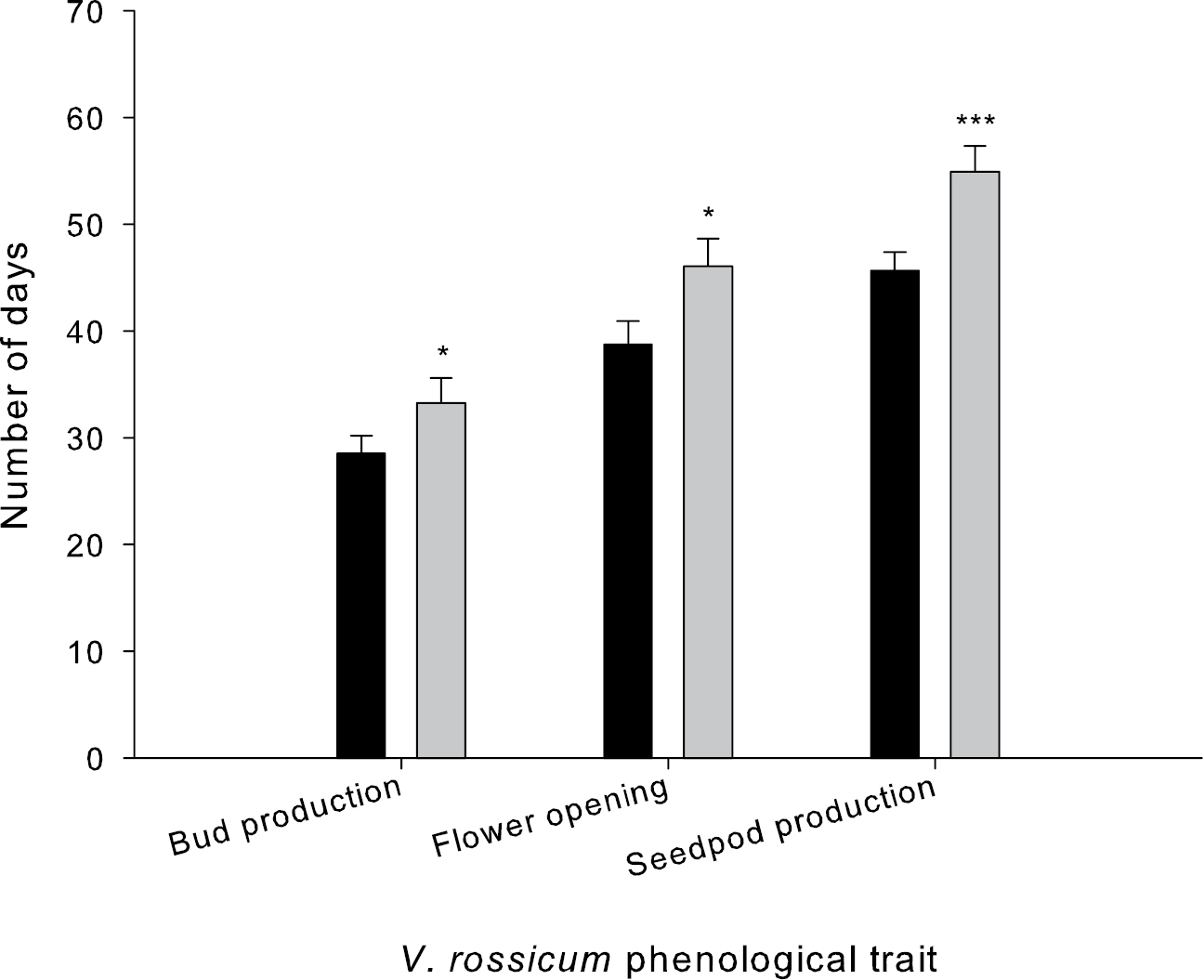
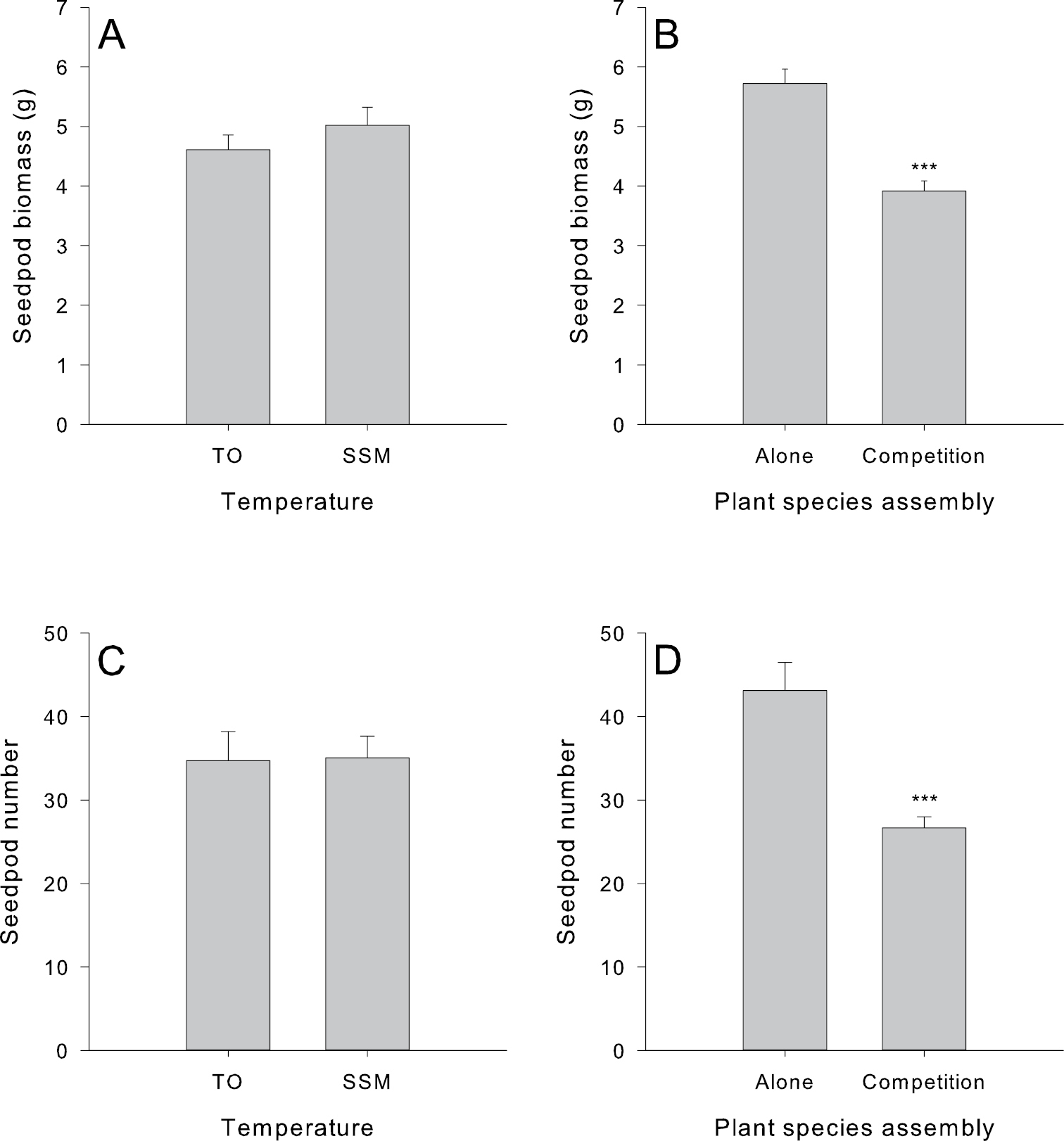
There were no significant differences in the reproductive phenology (i.e., presence of first flower bud, first flower opening and seedpod production) of Vincetoxicum rossicum grown either alone or with a competitor. In addition, we did not find a significant interaction between competition and temperature for any of these response variables. Temperature, however, had a significant effect on the reproductive phenology of Vincetoxicum rossicum (Fig. 1). Plants grown under SSM temperatures took significantly longer to produce flower buds (F1, 44 = 9.270, p = 0.00392), open flowers (F1, 44 = 11.040, p = 0.00180), and seedpod (F1, 44 = 19.778, p = 0.00006). These traits were delayed by an average of 8, 9 and 11 days, respectively, under the cooler SSM temperatures. By the end of the experimental growing season, however, all Vincetoxicum rossicum plants had produced the same biomass and number of seedpods containing mature seeds regardless of temperature treatment (Fig. 2A and C). Conversely, growing Vincetoxicum rossicum with a competitor caused significant reductions in the seedpod biomass of Vincetoxicum rossicum (F1, 36 = 42.812, p = 0.000001) and in the number of seedpods produced (F1, 36 = 30.73, p = 0.000003) (Fig. 2B and D). We did not detect a significant interaction between temperature and competition for seedpod biomass or number.
Number of days necessary for the production of buds, flowers and seedpods under TO (black bars) and SSM (grey bars) temperatures. Significant differences between temperature regimes for each phenological trait are indicated by * (p<0.05) ** (p<0.01) and *** (p<0.0001). Error bars represent the standard error of the mean (n = 24).
Effect of climatic temperature (i.e., Toronto (TO) and Sault Ste. Marie (SSM) and plant-plant competition (i.e., Vincetoxicum rossicum grown either alone or with Solidago canadensis (competition)) on seedpod biomass (A and B) and number (C and D) of Vincetoxicum rossicum at the end of the experiment. Significant differences between treatments are represented by *** (p<0.00001). Error bars represent the standard error of the mean (n = 24).
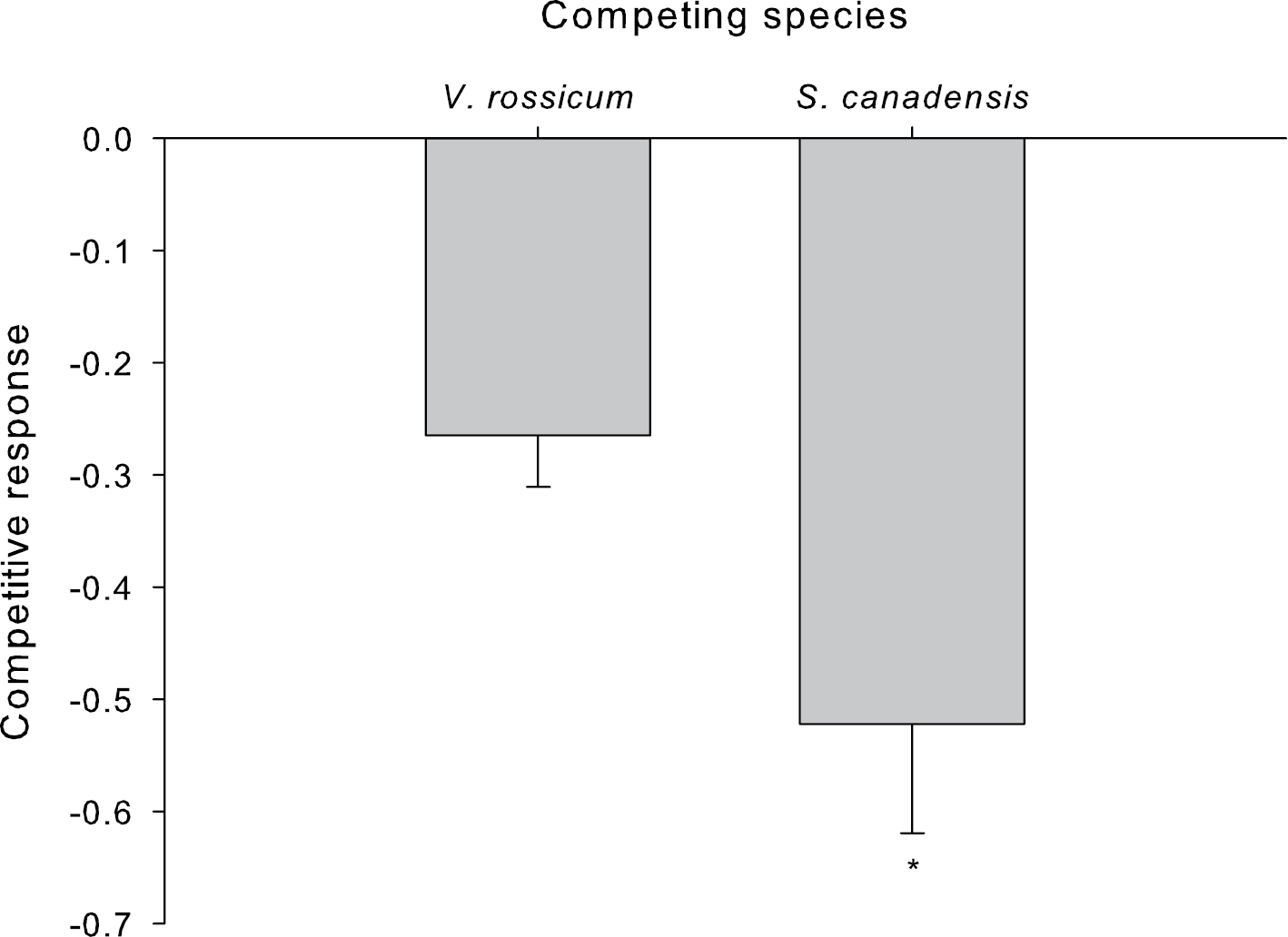
Competitive response (i.e., calculated by the ln of the relative yield, calculated by dividing the total biomass of an individual plant grown with a competitor by the average total biomass for that same plant species grown alone) of Vincetoxicum rossicum and Solidago canadensis relative to the presence of the competing species. Significant differences between species are indicated by an * (p<0.05). Error bars represent the standard error of the mean (n = 15 (Vincetoxicum rossicum; Solidago canadensis)).
Depending on the species, total plant biomass was affected by either temperature or competition. The total biomass of Vincetoxicum rossicum was not influenced by temperature but it was significantly affected by competition (F1, 35 = 8.3459, p = 0.007), and we did not detect a competition x temperature interaction (Table 2). Vincetoxicum rossicum was approximately two times more competitive than Solidago canadensis (F1, 25 = 4.60392, p = 0.042) (Fig. 3). Vincetoxicum rossicum plants grown with Solidago canadensis were 22% smaller than plants grown alone whereas Solidago canadensis plants were 37% smaller when competing with Vincetoxicum rossicum (F1, 32 = 12.914, p = 0.001) (Table 2; Fig 3). Growing temperature influenced the total biomass of Solidago canadensis. Plants grown under the cooler SSM conditions were 1.5 fold larger than those grown in the warmer temperature (F1, 32 = 6.1587, p = 0.018). There was no significant temperature x competition interaction.
Root: shoot ratio of Vincetoxicum rossicum was 1.4-fold higher in plants competing with Solidago canadensis as opposed to those grown alone (F1, 35 = 9.3602, p = 0.004) (Table 2). This ratio was not affected by temperature (Table 2) or the combination of temperature and competition (data not shown). Conversely, the root : shoot ratio of Solidago canadensis was not affected by any factor or their interaction.
Total biomass (g) and root : shoot ratio of Solidago rossicum and Solidago canadensis grown under Toronto (TO) and Sault Ste. Marie (SSM) temperature regimes and either alone or in competition with each other.
| Total biomass (g) | Root : shoot ratio | |||
|---|---|---|---|---|
| Vincetoxicum rossicum | Solidago canadensis | Vincetoxicum rossicum | Solidago canadensis | |
| TO | 23.56 ± 1.50 | 54.92 ± 4.90 | 2.05 ± 0.17 | 1.91 ± 0.18 |
| SSM | 22.75 ± 1.39 | 80.27 ± 8.99* | 1.93 ± 0.19 | 2.97 ± 0.37 |
| Alone | 25.27 ± 1.39 | 82.46 ± 8.55 | 1.72 ± 0.14 | 2.42 ± 0.29 |
| Competition | 19.68 ± 0.86** | 51.86 ± 5.23** | 2.41 ± 0.20* | 2.68 ± 0.42 |
For each species, statistically significant differences for each appropriate treatment factor are represented by * (p<0.05) and ** (p<0.001). Data are presented as mean ± standard error of the mean. (Vincetoxicum rossicum, temperature: n = 18 (TO), n = 21 (SSM); Vincetoxicum rossicum, plant species assembly: n = 24 (alone), n = 15 (competition); Solidago canadensis, temperature: n = 15 (TO), n = 21 (SSM); Solidago canadensis, plant species assembly: n = 21 (alone), n = 15 (competition)).
Temperature significantly affected the reproductive phenology of Vincetoxicum rossicum. Generally, phenological reproductive events took longer to occur under the cooler growing temperature conditions. This was expected as many plant species are known to accelerate their reproductive phenology when subjected to warming (
A plant’s northern range is determined by its capacity to overwinter and then produce viable seeds (
The native forb Solidago canadensis is highly abundant in disturbed areas in its native range and is an exotic invader in Europe and Asia (
We forced Vincetoxicum rossicum to compete against Solidago canadensis, which is highly abundant across the two climatic regions considered in this study. Competition between plants has been shown to reduce biomass, including allocation of biomass to reproduction (
Several factors were likely to have contributed to the competitive advantage of Vincetoxicum rossicum relative to Solidago canadensis in relation to total plant biomass. Plants were not limited by water, light or space aboveground, which suggests that most competition would occur belowground for limited nutrient resources, which were supplied in low concentrations throughout the course of the experiment. Vincetoxicum rossicum increased its root : shoot ratio when in competition with Solidago canadensis whereas Solidago canadensis showed no response. This response of Vincetoxicum rossicum is consistent with competitively-driven adaptive plasticity, which can be explained by the balanced resource hypothesis; plants allocate nutrients and energy for growth to the areas responsible for the acquisition of limiting resources (
Vincetoxicum rossicum has been shown to be dependent on the association with arbuscular mycorrhizal (AM) fungi (
Another possible reason for Solidago canadensis being a weaker competitor than Vincetoxicum rossicum could be that its timing for nutrient acquisition occurred later than that of Vincetoxicum rossicum’s. The two perennial species demonstrate different strategies; Vincetoxicum rossicum grows quickly early in the season whereas Solidago canadensis grows steadily over a longer period of time. A grassland study on invasion potential and resistance to invasion suggested that such resistance requires species that can establish and proliferate well, but also overlap the timing of their resource acquisition to that of the invading species (
In spite of a delay in reproductive phenology, the fitness of Vincetoxicum rossicum does not appear to be limited by cooler growing season temperature regimes found outside its immediate current distribution range in North America. Competition resulted in reductions in the fitness and total biomass of Vincetoxicum rossicum regardless of climatic temperature. However, the relative reductions in total biomass were greater for the competing native species Solidago canadensis.
This research was funded in part by the Invasive Species Centre. LS was funded through the internship program of the North Ontario Heritage Fund Corporation. PMA was funded by a Research Chairship Grant from the Ontario Ministry of Natural Resources to Algoma University and by a Natural Sciences and Engineering Research Councilof Canada(NSERC) Discovery Grant. We thank Dr. Michael Irvine for his advice and the Ontario Forestry Research Institute staff for technical support and access to the controlled environment units.