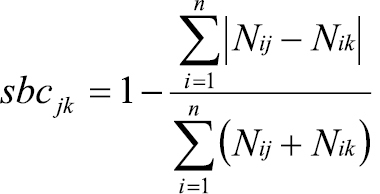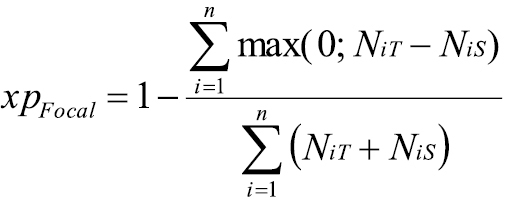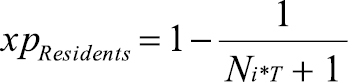| Taxon names | Citations | Turn highlighting On/Off |







(C) 2013 Wolf-Christian Saul. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Citation: Saul W-C, Jeschke JM, Heger T (2013) The role of eco-evolutionary experience in invasion success. NeoBiota 17: 57–74. doi: 10.3897/neobiota.17.5208
Invasion ecology has made considerable progress in identifying specific mechanisms that potentially determine success and failure of biological invasions. Increasingly, efforts are being made to interrelate or even synthesize the growing number of hypotheses in order to gain a more comprehensive and integrative understanding of invasions. We argue that adopting an eco-evolutionary perspective on invasions is a promising approach to achieve such integration. It emphasizes the evolutionary antecedents of invasions, i.e. the species’ evolutionary legacy and its role in shaping novel biotic interactions that arise due to invasions. We present a conceptual framework consisting of five hypothetical scenarios about the influence of so-called ‘eco-evolutionary experience’ in resident native and invading non-native species on invasion success, depending on the type of ecological interaction (predation, competition, mutualism, and commensalism). We show that several major ecological invasion hypotheses, including ‘enemy release’, ‘EICA’, ‘novel weapons’, ‘naïve prey’, ‘new associations’, ‘missed mutualisms’ and ‘Darwin’s naturalization hypothesis’ can be integrated into this framework by uncovering their shared implicit reference to the concept of eco-evolutionary experience. We draft a routine for the assessment of eco-evolutionary experience in native and non-native species using a food web-based example and propose two indices (xpFocal index and xpResidents index) for the actual quantification of eco-evolutionary experience. Our study emphasizes the explanatory potential of an eco-evolutionary perspective on biological invasions.
Alien species, ecological novelty, ecological similarity, introduced species, invasibility, invasiveness, naïveté, non-indigenous species
A large number of hypotheses about the mechanisms that determine the success or failure of biological invasions have been proposed (reviews in
With this conceptual paper we aim at contributing to this important development. We suggest that adopting an eco-evolutionary perspective on invasions is a promising approach to achieve a broader conceptual synthesis in invasion ecology (cf.
During evolution, species adapt to biotic interactions in their native environment. They thereby accumulate what we propose to term ‘eco-evolutionary experience’ in dealing with these interactions. We hypothesize that this inherited experience – possibly complemented by experience acquired during an individual’s lifetime (e.g. predators getting better at capturing prey during successive encounters) – ultimately determines the species’ proficiency to survive and prosper within new ecological contexts, as for example when invasions take place. For an introduced species, the biotic community in its exotic range may differ fundamentally from the one in its native environment. Biotic interactions that evolutionarily shaped the introduced species in its native environment may become interrupted (
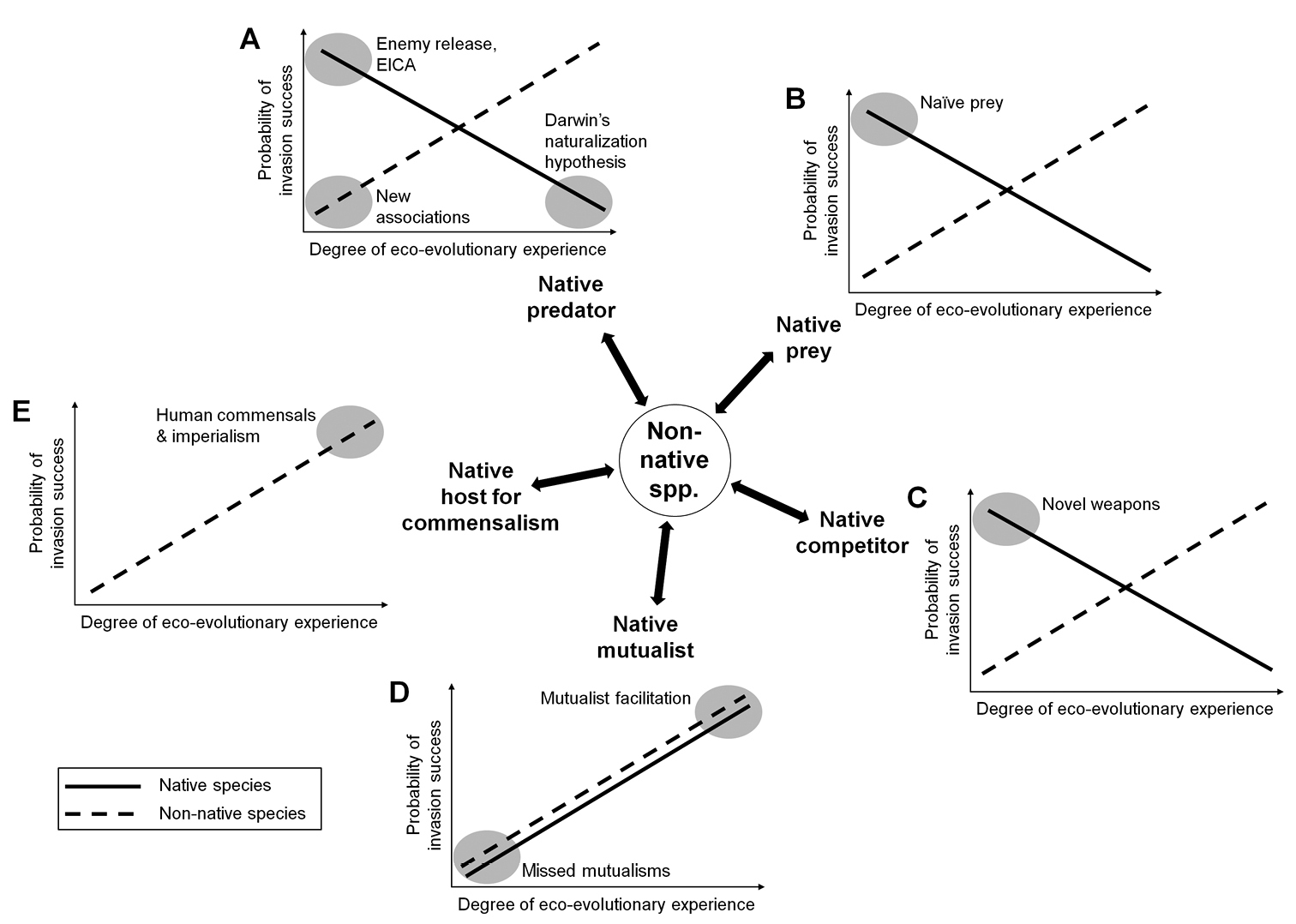
Figure 1 illustrates a conceptual framework to explain variation in invasion success based on the concept of eco-evolutionary experience. The framework consists of five hypothetical scenarios, corresponding to five major types of ecological interaction: the introduced species acting as prey (Fig. 1A), predator (including herbivores, parasites, and parasitoids; Fig. 1B), competitor (Fig. 1C), mutualist (Fig. 1D) or commensal (Fig. 1E). The graphs presented in each scenario are speculative, their exact shape still to be substantiated with empirical data in future studies. However, the scenarios formulate our generalized hypotheses about the relationship between the eco-evolutionary experience in the interacting introduced and native species on the one hand and the relative probability of the respective invasion to succeed on the other: For predator-prey and competitive interactions, the probability of a successful invasion is likely to be higher with a low degree of applicable eco-evolutionary experience in the native species and a high degree in the non-native species (Fig. 1A, B, C). Widely known examples where these circumstances likely apply include the invasion of purple loosestrife (Lythrum salicaria) in North America where it lacks herbivorous enemies that feed on it in its native range (
Framework of five hypothetical scenarios about the influence of eco-evolutionary experience in the non-native (dashed line) and native species (solid line) on the relative probability of invasion success, according to the type of ecological interaction (A/B: predator-prey, C: competition, D: mutualism, E: commensalism). In general, lower native experience (except in mutualistic interactions) and higher non-native experience is likely to be advantageous for invasion success. Shaded ovals exemplarily indicate parts of the framework covered by major hypotheses in invasion ecology that implicitly share a reference to the importance of evolutionary legacy for invasion success (see main text and Appendix I for details and references).
Notably, several major invasion hypotheses can be integrated into this framework. From an eco-evolutionary viewpoint, it becomes apparent that they actually share an implicit reference to the role of evolutionary legacy in invasion success. This includes such often-cited hypotheses as ‘enemy release’ (
By adopting an explicit eco-evolutionary perspective, the framework provides a basis for interrelating the hypotheses (as defined in Appendix I) and conclusions based on them, but it also highlights their shortcomings: the hypotheses of enemy release, EICA, Darwin’s naturalization hypothesis, naïve prey and novel weapons consider the degree of experience only on the native species’ side (Appendix I: a, b, d, e, f), while new associations, missed mutualisms, and the human commensals and imperialism hypothesis focus on the non-natives’ experience (Appendix I: c, g, i). Only the mutualist facilitation hypothesis at least implicitly considers both sides (Appendix I: h). Thus, these invasion hypotheses emphasize either the invasibility of native communities or the invasiveness of non-native species and neglect that the outcome of an invasion is probably influenced by the degree of applicable eco-evolutionary experience on both interacting sides (cf.
Clearly, in connection with the framework presented here, practicable approaches to actually quantify eco-evolutionary experience are needed. Such approaches can build on the general assumption that more of the eco-evolutionary experience in species (native or introduced) will be applicable to a new interaction setting if that setting is ecologically similar to previous interactions. In other words, the degree of ecological similarity between new and previous interaction settings may be taken as a proxy for the degree of applicable eco-evolutionary experience in native and non-native species.
Ecological similarity of species is often assumed to be positively correlated with the taxonomic or phylogenetic relatedness between them (e.g.
Our approach for quantifying eco-evolutionary experience of introduced and native species assesses the ecological similarity of the ecological interaction settings these species are part of before and after the invasion. Such comparisons can be done for any ecological network, e.g. plant-pollinator networks, seed-dispersal interactions, host-parasite systems or food webs. We here present an example for a quantification routine based on food webs (summarized in Appendix II), which covers predator-prey, competitive, and indirect mutualistic interactions (e.g. a predator and a primary producer indirectly benefitting from each other as the predator feeds on the herbivore that consumes the primary producer). We compare the food webs of the original ‘source’ area and a new ‘target’ area of the introduced species (hereafter called the ‘focal species’) regarding the occurrence and occupancy (in terms of number of species) of ecological guilds. Note that the term ‘guild’ as we use it here is not restricted to referring exclusively to “a group of species that exploit the same class of environmental resources in a similar way” (
In order to assess the experience of the focal species after its introduction to a target area, we compare the interactions in the food webs of these two areas from the perspective of the focal species (steps 1 to 4 in Appendix II). Both food webs will be composed of different trophic levels, each of which may contain species of different ecological guilds. For simplicity, we restrict our analysis to direct interactions and single-step indirect interactions (i.e. including one intermediate species as for example in exploitative competition) of the focal species with resident species (step 1 in Appendix II). These interactions can be assumed to have the most immediate consequences for the invasion success of the focal species. Separately for each type of interaction (i.e. the focal species acting as prey, predator, competitor or indirect mutualist), and for both the source and target area, the respective interaction partners are classified into their ecological guilds and the members of each guild are counted (steps 2 and 3 in Appendix II). In this way, we obtain datasets for each type of interaction, with species numbers per guild in both the source and target area (see exemplary Table A in Appendix II).
To actually calculate the eco-evolutionary experience of the focal species (step 4 in Appendix II), we need an index of similarity. The Bray-Curtis similarity index (sbc) is often used in ecological studies when comparing the species composition of different samples, e.g. community samples:
where n is the total number of species considered, and Nij and Nik represent the number of individuals of species i in the samples j and k, respectively. Absolute abundance differences in all species are summed up in the numerator and standardized by the total number of individuals in all species from both samples in the denominator. However, while this index provides some grasp on the absolute difference between the samples, it does not consider the direction of change in numbers. But this is important from an eco-evolutionary perspective in the invasion context: for the focal species, it is decisive whether it encounters more or fewer interaction partners from particular guilds in the target area than in the source area. We thus adapted the Bray-Curtis index to account for this specific need. The new index is an index of experience rather than just similarity. We thus call it ‘xpFocal index’:
where n is the total number of guilds considered, and NiS and NiT represent the number of species in guild i in the source (S) and target (T) area, respectively, that interact with the focal species. Values of xpFocal range between 0 (no applicable experience in the target area) to 1 (maximum applicable experience). By considering not only the presence or absence of guilds but also how numbers of species occupying these guilds differ between source and target area, the xpFocal index accounts for trait differences on the guild level as well as species level. In contrast to the Bray-Curtis index, however, the xpFocal index only considers those differences in the number of guild members where NiS<NiT by introducing the ‘max’ term in the numerator. From the perspective of the focal species, these are the relevant differences between the source and target area, because a larger number of interaction partners of a guild in the target area compared to the source area implies a reduced (or even absent) eco-evolutionary experience of the focal species in the new interaction setting.
This is obvious in cases where the focal species meets interaction partners of a guild in the target area that was entirely absent in the source area (i.e. when NiS=0 and NiT>0), being then unable to count on applicable eco-evolutionary experience for these new interactions. But reduced experience is also expected when the focal species interacts with species even of a familiar guild if they occur in larger numbers in the target area as compared to the source area (NiS<NiT). This is reasonable to assume because also species of the same guild differ from each other. Although these differences are relatively small (otherwise the species would be classified into different guilds), they can still be relevant for the focal species. Thus, the more interacting species exist in the target area in comparison to the source area (i.e. the larger NiT is in relation to NiS), the higher is the probability that the focal species will have to respond to unknown ecological traits, and the lower is its experience in the target area. By contrast, the probability of having to respond to unfamiliar ecological traits of species of a particular guild is low when the focal species has already interacted with a larger number of species from that guild in the source area than in the target area. Our model makes the simplifying assumption of a threshold where the focal species has the maximum eco-evolutionary experience with the new interaction setting (xpFocal=1) when it has interacted with at least as many species in each guild in the source area as it encounters in the target area (i.e. if NiS≥ NiT). In future studies, alternative formulations without such a threshold may be explored.
To a certain degree, the xpFocal index allows reduced experience with members of a particular guild to be compensated by experience in the same type of interaction with species of other guilds. For instance, in predator-prey interactions the focal species may not be familiar with predators of a particular guild in the target area, but may also not be entirely naïve because of having evolved in its source area in the presence of predators at least from other guilds. However, under the assumptions of the xpFocal index, such ‘unspecific’ experience with a type of interaction (in this example ‘predation’) will not completely offset missing experience with a particular guild.
In order to assess the experience of the resident species community facing a new introduced species, we first determine the focal species’ guilds for each type of interaction, i.e. when it may act either as a predator, prey, competitor or indirect mutualist. We then count the number of resident species that are already present in these specific guilds in the target area (see step 5 and exemplary Table B in Appendix II). Finally, by calculating the following ‘xpResidents index’ separately for each type of interaction (step 6 in Appendix II), we can assess, in a first approximation, how much experience native species have with the focal species:
where Ni*T is the number of resident species in the same guild (i*) as the focal species in the respective type of interaction. The fraction in this index provides an estimate how ecologically ‘novel’ the focal species is for the resident community. The maximum novelty of the focal species (i.e. the least experience in resident species) can be expected if no resident species are present in the focal species’ guild before the invasion event. The novelty of the focal species gradually decreases with an increasing number of resident species that are in the same guild as the focal species. Subtracting the fraction from 1, we obtain the eco-evolutionary experience of the resident species community (xpResidents), with values ranging again between 0 (no applicable experience of resident species with the focal species) to 1 (maximum applicable experience).
Having thus calculated both the eco-evolutionary experience of the focal species (xpFocal) and the experience of the resident species community (xpResidents) for different types of interaction, we can return to the framework in Fig. 1 and estimate the probability of the invasion to succeed.
In the previous chapters, we introduced a framework that – by adopting an eco-evolutionary perspective – integrates so far unrelated approaches for explaining biological invasions, and we drafted a routine to quantify eco-evolutionary experience, which is the key variable in this framework. It has to be emphasized again that the framework is of conceptual nature. For instance, the assumed relationship between eco-evolutionary experience and invasion success has to be substantiated with empirical data beyond the hypothetical graphs presented in Fig. 1. Furthermore, the quantification routine makes several simplifying assumptions that have to be kept in mind for an appropriate interpretation:
- Species are adapted to virtually all of their biotic interactions in the source area, which constitutes the inherited eco-evolutionary experience that may matter in ecologically similar communities in the target area. In reality, species are not necessarily adapted to all interactions, e.g. due to weak selection pressure, evolutionary trade-offs, or gene flow. Furthermore, we assume there is no significant intraspecific variation in species traits, e.g. among different populations of the same species.
- Adaptation has no costs. Consider, for example, two focal species that face a single predator species of guild R3 from the example in Appendix II in their respective target areas. For both of them, we would calculate xpFocal=1 if during their evolution in the source area they adapted to at least one predator species of the guild R3. The same xp value would be computed even if one of the focal species had adapted to additional predator species. In reality, such ‘over-adaptation’ would probably have generated costs, which could imply disadvantages when compared to the other focal species, but in our model it does not translate into a lower probability of invasion success.
- All interactions are assumed to be equal in strength and frequency. For instance, no distinction is made between generalists and specialists, or whether the focal species interacts in the target area with exactly the same species as in the source area or just with a member of the same guild.
- There is no amplifying effect within interaction types: an interaction partner is counted only once in each type of interaction, even if it maintains more than one ‘connection’ with the focal species within that interaction type (e.g. when competing with the focal species for several prey species).
- As mentioned above, only a subset of all interactions in the studied food webs is included in the analysis, i.e. direct and single-step indirect interactions, and the number of interacting partners in each guild depends on the particular guild definition chosen.
On a side note, we focused in this paper on novel biotic interactions that may influence invasion success in order to demonstrate the usefulness of an eco-evolutionary perspective in invasion research. This is not to argue, of course, against the substantial effect that other factors may have on invasion success as well. The significant influence of abiotic conditions has been indicated, for instance, by studies on the effect of climate change (
We believe that the indices proposed here (xpFocal and xpResidents) constitute an important first step towards an efficient quantitative estimate of the influence of species’ evolutionary legacy on the success of biological invasions. A particular strength of this approach lies in its high flexibility: it allows considering not only food webs but also other ecological networks; different kinds of ecological groupings (ecological guilds, functional groups etc.) can be used; and it is applicable to all living organisms across taxonomic boundaries (e.g. plants and animals alike).
From an applied perspective, the further development of the framework and quantification routine to include less simplifying assumptions is certainly highly desirable and a stimulating research perspective. An important next step is to actually test the usefulness of our framework and the quantification routine for empirical case studies. Also, it should be investigated how the various xp values computed for the different types of interaction can best be integrated to provide an overall estimate of invasion probability. This could, for instance, be done by reducing complexity (and potential inconsistencies) considering only the most important type(s) of interaction in the respective case study, or it could comprise the development of a single, combined xp value.
An integrative and comprehensive conceptual treatment of conclusions derived from findings in both ecological and evolutionary research is still hard to find in invasion ecology. However, as we have outlined above, such an eco-evolutionary perspective would not merely add parenthetical historical information but would increase our potential to uncover invasion patterns. Our framework provides the means for interrelating seemingly isolated ecological invasion hypotheses by identifying implicit eco-evolutionary assumptions (Fig. 1, Appendix I). The framework thus helps to synthesize the conclusions drawn from these hypotheses, providing a stronger basis for a more general understanding of invasion mechanisms and reasons for variation in invasion success. It ties in with the idea of a ‘hierarchy of hypotheses’ (
The framework generates new, although still very general conceptions on how invasion success depends on eco-evolutionary experience and emphasizes the importance of considering both interacting sides simultaneously: native and non-native species. It also takes into account that non-native species may take up different ecological roles in the exotic range and allows differentiated conclusions for the major types of ecological interactions that may be affected by the invasion.
We believe that the conceptual insights that can be derived from our framework and the quantification routine can be of significant help to guide future research. Ultimately, this research may lead to effective management measures to prevent the introduction of species that seem particularly ‘risky’ for a specific target area, or to adopt appropriate mitigation or restoration measures.
We like to thank Ludwig Trepl who inspired our initial discussions about the influence of evolutionary legacy on invasion success. Earlier drafts of the manuscript greatly benefited from comments provided by Johannes Kollmann, Robert Colautti and anonymous reviewers. JMJ acknowledges financial support from the Deutsche Forschungsgemeinschaft (grant JE 288/4-1).
The shared eco-evolutionary basis of major hypotheses in invasion ecology
The concept of ‘eco-evolutionary experience’ posits that biotic interactions maintained during the evolutionary history of species influence the outcome of interactions between native and introduced species in present times, i.e. (a) the invasion success of the introduced species and (b) the responses of natives. Several major hypotheses for explaining invasion success can be directly related to this concept based on their implicit reference to the logical consequence of a species being introduced into an area where it has not evolved (for references see main text):
a) Specialized, i.e. eco-evolutionarily highly experienced native enemies of the introduced species may be missing (‘enemy release hypothesis’).
b) Reduced predation due to inexperienced native predators (herbivores) may allow the introduced species to allocate more resources to traits that increase its competitive abilities (‘evolution of increased competitive ability hypothesis’).
c) The introduced species may be inexperienced with native enemies and may therefore lack appropriate defence mechanisms (‘new associations hypothesis’).
d) Introduced species with close relatives in the target area may be less successful because native predators may already be experienced with native congeneric prey species (‘Darwin’s naturalization hypothesis’).
e) Native prey species may be unprepared, i.e. inexperienced for effectively countering novel predatory behaviour of an introduced species (‘naïve prey hypothesis’).
f) Native species may not be adapted to, i.e. may be inexperienced with specialized competitive strategies of the introduced species (‘novel weapons hypothesis’).
g) Mutualistic interactions may fail to develop because of missing experience between native and non-native species (‘missed mutualisms hypothesis’).
h) Mutualistic interactions between a native and non-native species may be possible, provided that the degree of experience is high enough in both interaction partners (‘mutualist facilitation hypothesis’).
i) Species that have evolved a strong commensal affiliation to humans may benefit from this eco-evolutionary experience when introduced to areas dominated by humans. This may be especially true for Eurasian species: they coevolved with Europeans and their plants, pathogens and livestock, which were dispersed all over the world during the European Imperialism period (‘human commensals and imperialism hypothesis’).
Routine for the quantification of eco-evolutionary experience: a food web-based example
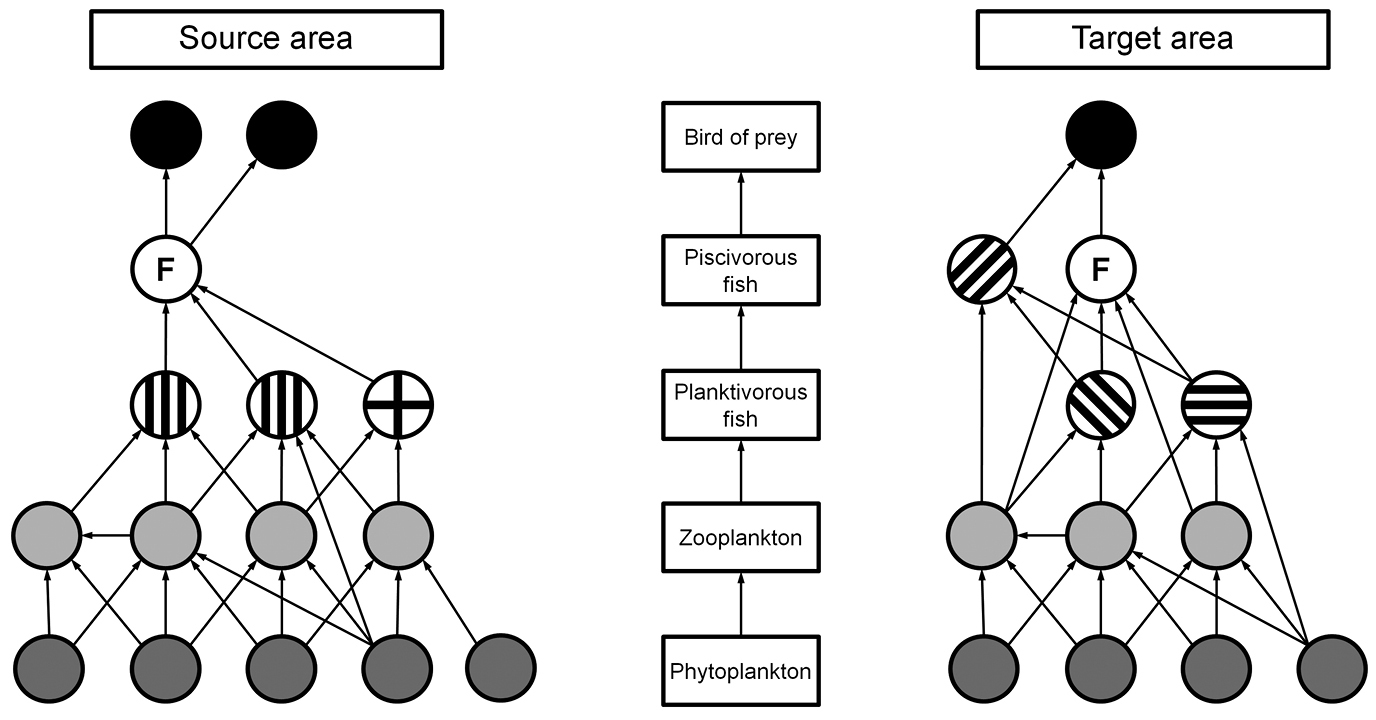
1. Identify direct interactions and single-step indirect interactions (i.e. including one intermediate species) of the focal species in the food web of its source area and in the food web of the (potential) target area (see example in Fig. A).
2. Define ecological guilds (or other appropriate ecological groupings) for each type of ecological interaction (focal species acting as prey, predator, competitor or indirect mutualist). Assign the focal species and its interaction partners in the source and target area to the ecological guilds.
Quantification of the focal species’ eco-evolutionary experience (xpFocal):
3. Determine the number of species that interact with the focal species per ecological guild in the source and target area, separately for each type of interaction (Table A).
4. Calculate the xpFocal index (Eq. 2) for each type of interaction, obtaining the eco-evolutionary experience of the focal species regarding its interaction with resident species in the food web of the target area.
Quantification of the resident species’ eco-evolutionary experience (xpResidents):
5. Determine the number of resident species in the target area that are members of the same ecological guild as the focal species (regardless if they interact with the focal species or not), separately for each type of interaction (Table B).
6. Calculate the xpResidents index (Eq. 3) for each type of interaction, obtaining the eco-evolutionary experience of the resident species community regarding its interaction with the introduced focal species.
Hypothetical food webs in freshwater lakes in source and target area. Circles represent species (F = focal species), different shading and patterning indicate different guilds (see steps 2 and 3).
Numbers of species per guild that interact with the focal species in the food webs of the source and target area (taken from Fig. A), and the respective eco-evolutionary experience of the focal species (xpFocal) in the target area (R1-R5: predator guilds, P1-P5: prey guilds, C1-C5: competitor guilds, M1-M5: mutualist guilds).
| Type of interaction Interaction partners of the focal species |
No. of species in guild i in source area S | No. of species in guild i in target area T | xpFocal | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| i= | R1 | R2 | R3 | R4 | R5 | R1 | R2 | R3 | R4 | R5 | |
| Predators | 2 | - | - | - | - | 1 | - | - | - | - | 1.00 |
| i= | P1 | P2 | P3 | P4 | P5 | P1 | P2 | P3 | P4 | P5 | |
| Prey | 2 | 1 | - | - | - | - | - | 1 | 1 | 2 | 0.43 |
| i= | C1 | C2 | C3 | C4 | C5 | C1 | C2 | C3 | C4 | C5 | |
| Competitors | - | - | - | - | - | 1 | 1 | 1 | - | - | 0.00 |
| i= | M1 | M2 | M3 | M4 | M5 | M1 | M2 | M3 | M4 | M5 | |
| Indirect mutualists | 4 | 1 | - | - | - | 3 | 4 | - | - | - | 0.75 |
Number of resident species in the target area that are members of the same guild as the focal species (note that species numbers are exemplary and not directly deducible from Fig. A), and the respective eco-evolutionary experience of the native community (xpResidents) with the focal species.
| No. of resident species in same guild as the focal species | xpResidents | |
| Predators | 1 | 0.50 |
| Prey | 0 | 0.00 |
| Competitors | 3 | 0.75 |
| Indirect mutualists | 2 | 0.67 |