(C) 2013 Martin Hejda. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Citation: Hejda M (2013) Do species differ in their ability to coexist with the dominant alien Lupinus polyphyllus? A comparison between two distinct invaded ranges and a native range. NeoBiota 17: 39–55. doi: 10.3897/neobiota.17.4317
The community-level impacts of invasive plants are likely to vary depending on the character of native species of the target communities and their ability to thrive within the stands of the dominant alien invader. Therefore, I examined the response of native species richness to the cover of the dominant alien Lupinus polyphyllus in two distinct invaded ranges: Czech Republic (Central Europe) and New Zealand. I compared the relation between native species richness and the cover of the dominant alien Lupinus polyphyllus with that in its native range, Pacific Northwest, USA.
In the native range, I found no response of native species richness to the cover of Lupinus polyphyllus. In the Czech Republic (central Europe), the richness of native species related to it negativelly, but the relation was only marginally significant. Contrary to that, the richness of species native to New Zealand related to the cover of Lupinus polyphyllus strongly negatively and the negative relation was significantly stronger than that of species native to Europe.
Of the two invaded ranges, species native to New Zealand have been documented to be much more vulnerable to the conditions associated with the invasion and dominance of Lupinus polyphyllus, compared to species native to central Europe. This principle has been shown both across these two invaded ranges and in New Zealand, where the aliens of european origin successfully coexist with the dominant invasive alien Lupinus polyphyllus. Similarly, species in the native range of Lupinus polyphyllus showed no relation to its cover, indicating their ability to thrive even in dense stands of this dominant species.
Lupinus polyphyllus, invasive alien species, native range, invaded range, coexistence of species, diversity, dominance
Invasions of alien plant species have become widely recognized as one of the major human-induced changes, affecting the whole biosphere at the global scale (
It has been documented that the character of the recipient community co-determines the magnitude of the alien´s impact on diversity and, therefore, a single invasive species can have different impacts in different types of invaded communities (
Aliens can alter conditions on large areas, such as when a tree species invades a formally treeless environment, as documented on Galápagos by
This paper aims to test the ability of native species to coexist with the dominant alien Lupinus polyphyllus and attempts to test the following hypotheses:
Do species of native and invaded ranges differ in their ability to thrive on sites with high dominance of Lupinus polyphyllus?
Do native species of different invaded ranges (Czech Republic, central Europe; New Zealand) differ in their ability to coexist with the dominant invasive alien Lupinus polyphyllus?
Lupinus polyphyllus is a 0.7 – 1.2 m tall, robust, rhizomatous perennial native to the Pacific Northwest of USA. In its native range, Lupinus polyphyllus has been reported to grow in wet montane meadows, along streams, but also as a viatic weed. Despite Lupinus polyphyllus being poisonous, there have been attempts to use it as fodder plant and low-alcaloid varieties have been introduced (
For ornamental and landscape purposes, Lupinus polyphyllus was intentionally introduced to many regions of the world and has become invasive in central and northern Europe, Southern island of New Zealand and Tasmania. In the Czech Republic, Lupinus polyphyllus invades wet montane and submontane meadows, river banks and forest edges (Slavík et al. 1995). Both in its native and invaded ranges, the occurrence of Lupinus polyphyllus seems to respond to human-induced disturbance positivelly and it often grows along roads and in other anthropogenically impacted places (see for example Valtonen et al. 2003). At the same time, Lupinus polyphyllus is apparently able to colonize even rather extreme sites, with rocky and unstable substrates, periods of stress and / or low nutrient levels. This can be seen in New Zealand especially, where Lupinus polyphyllus often colonizes frequently disturbed and rocky terraces of montane and submontane rivers (
The community-level impacts of Lupinus polyphyllus as an invasive alien have been studied in both Europe (
The invasion of lupins is apparently promoted by the intentional introduction of generalist pollinators, such as bees or bumble-bees (
I carried out this comparative study in the native range of Lupinus polyphyllus, which is the Pacific Northwest of USA, and two distinct invaded ranges (Southern Island of New Zealand and Czech Republic, central Europe).
In the native range, I sampled the data in the states of Washington and Oregon, USA. In Washington, the data were clustered around Mt. Rainier and Mt. Adams, while in Oregon, I sampled the data in the Columbia river Gorge around Bridal Veils. In New Zealand, I sampled the data on riparian meadows around the Waimakariri river, Arthur´s Pass National park and around Eglington river, Fjordland National Park, Southern Island. In the Czech Republic, I sampled the data in Jizerské hory (NE Bohemia) and Slavkovský les (W Bohemia) natural and landscape reserves and around the town of Průhonice (central Bohemia). In all of these three ranges, I collected the data in mesic to wet meadows. All areas revealed relatively high precipitation and were not prone to summer drought periods.
It was not possible to locate the vegetation plots randomly, mainly because of spatially autocorrelated distribution of Lupinus polyphyllus in the invaded ranges. In the Czech Republic and especialy in New Zealand, Lupinus polyphyllus was found to be excessively abundant in some areas, whereas it was absent in other areas. This type of strongly autocorrelated spatial distribution leads to the plots being clustered in the areas, where Lupinus polyphyllus was abundant and where it was observed to massively invade near-natural communities and compete with native species. The aim was to sample vegetation with a wide scale of Lupinus polyphyllus´ cover (dominance) in each area of its occurence. The GPS coordinates of plots are available in Appendix I.
In all of the three ranges, I collected a dataset of 40 plots of an area of 2 × 2m with varying cover of Lupinus polyphyllus. I used the fine-scale plots because the aim of the project was to test the ability of species to thrive within the dense and homogenous stands of Lupinus polyphyllus. On larger scales, the stands of Lupinus polyphyllus tend to be patchy rather than homogenous, so the results would be biased by native species´ growing in these empty patches rather than within the stands of Lupinus polyphyllus. I recorded the present species and estimated their relative abundances on a percentage scale. Altogether, I sampled 120 plots of communities with Lupinus polyphyllus from the three ranges together, plus 40 plots with the alien dominant Hieracium pilosella agg. and 40 plots with the alien dominant Anthoxantum odoratum in New Zealand, which makes 200 plots altogether. I estimated the dominance of lupins (and other alien dominants in New Zealand) as its percentage cover, which can be assumed to be a quick and easy to get proxy for biomass. At fine spatial scale, I selected sites with comparable conditions (light, stability and moisture of substrate, degree of ruderalization), in order to minimize the likelihood that the cover of lupins would be confounded with other basic environmental factors, biasing the results. I found the taxonomy of Lupins to be very complicated in the native range (Pacific Northwest, USA) and I had to exclude several plots from the dataset, leading to merely 22 plots from the native range. Lupins on the excluded plots were probably hybrids between Lupinus polyphyllus and other related species, such as Lupinus latifolius and Lupinus burkei. Although these hybrids between Lupinus polyphyllus and closelly related species were of similar appearance with robust stature and rhizomatous growth, they could have impacted the coexisting species differently, due to differences in e. g. nitrogen fixation rate or production of allellopathic compounds. For these reasons, I decided to keep the taxonomic delimitation of Lupinus polyphyllus as consistent as possible across the ranges where the plots were sampled, however, leading to just 102 plots with Lupinus polyphyllus used in the data analysis, compared to the originally intended 120.
I tested the response of species richness to the cover of lupins (and to the cover of Anthoxantum odoratum and Hieracium pilosella agg. in New Zealand) using Pearson correlations and the linearity of these relations using regression models. Further, I tested the differences in the response between various subsets of species (native to USA, native to Europe and native to New Zealand) by the mixed-effect analysis of covariance (
I used the ratios in the numbers of species between each plot and the most diverse plot sampled within the category of plots (USA, Czech Republic, New Zealand) as response variables. The plot with the maximum species richness within a particular category had an importance value of 1, while the other plots from this subgroup had importance values between 0 – 1, when the zero value says no species were recorded besides Lupinus polyphyllus and the value of 1 says the plot harboured the same number of species as the plot with maximum species richness within that category. I did this because plots from the three ranges differed in native species richness substantially, with the invaded stands from New Zealand harbouring much less native species compared to the stands in either the Czech Republic or in the native range, USA. In other words, the difference of 5 native species between the least and most invaded stands represents a very different portion of native species richness recorded in New Zealand and in the Czech Republic. Therefore, I considered these ratios, representing portions of species thriving on a particular plot from the maximum sampled within each category of plots, as more relevant than simple numbers of species.
A separate mixed-effect regression model was created to test the response of native vs. alien species (of european origin exclusively) to the cover of Lupinus polyphyllus in New Zealand. In this regression model, the identity of sampling areas in New Zealand (Arthur Pass, Eglington River Valley) was the random effect, while the cover of Lupinus polyphyllus was the fixed effect. The ratios between the numbers of native / alien species were used as the response variable in this model in order to reflect the autocorrelation between the alien and native species richness, recorded on a single vegetation plot.
I tested the significance of particular terms via deletion tests, when the growth of unexplained variance following the removal of a particular term (main effect or interaction) was tested using F-tests in case of regression models and Chi2 tests in case of the mixed - effect models. I performed all univariate analyses in R software (
I performed a direct gradient analysis (CCA) to detect the response of community´s species composition to the abundance (cover) of Lupinus polyphyllus. Before doing the direct gradient analyses, I performed an indirect gradient analysis (DCA) to check for the heterogeneity within the dataset and to decide whether to use a linear or unimodal approximations (
In all of the three ranges (USA, Czech Republic, New Zealand), I sampled plots with the cover of Lupinus polyphyllus of up to 90% (Appendix II).
In the native range (Pacific NW USA), I recorded 112 native species and 52 aliens of European origin exclusively in the vegetation with Lupinus polyphyllus. In the Czech Republic, 120 native species were recored in the vegetation invaded by Lupinus polyphyllus along with 6 aliens, with origins in Europe or SW Asia. In New Zealand, I recorded only 33 native species within the stands of Lupinus polyphyllus, but also 52 alien species, exclusively of european origin (Appendix I and II).
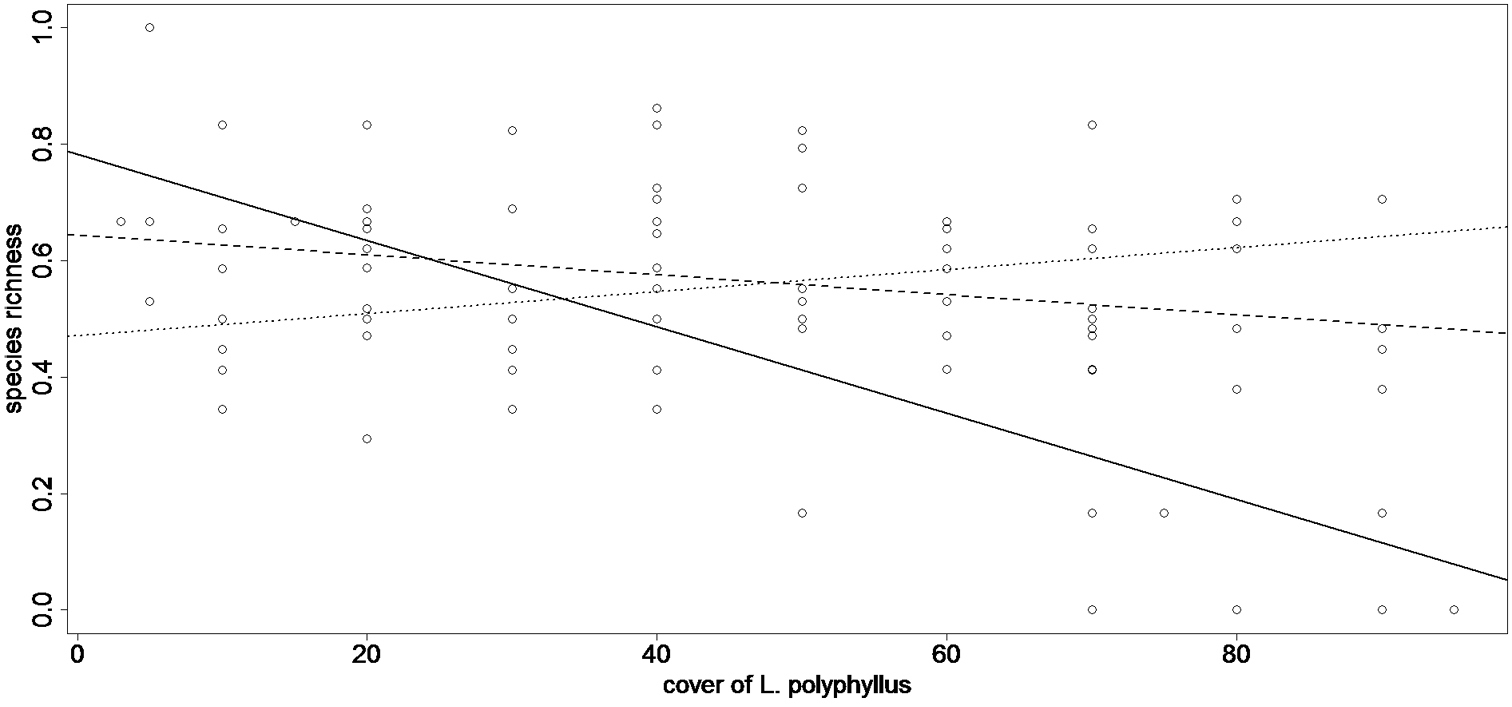
In the Czech Republic, native species´ richness responded to the cover of Lupinus polyphyllus negativelly (r = -0.294 – Table 1, Fig. 1), but the relationship was only marginally significant (p = 0.065 – Table 1, Fig. 1). In New Zealand, native species responded to the cover of Lupinus polyphyllus negativelly (r = -0.757, p < 0.001 Table 1, Fig. 1). Species of european origin growing as aliens in New Zealand did not respond to the cover of Lupinus polyphyllus (r = -0.160, p = 0.324 – Table 1), nor did species native to New Zealand respond to the cover of other invaders of the target communities (Hieracium pillosella agg. – r = -0.104, p = 0.523, Anthoxantum odoratum – r = 0.070, p = 0.666 – Table 1). I detected no relation between species richness and the cover of Lupinus polyphyllus in its native range, Pacific Northwest, USA (r = 0.308, p = 0.163 – Table 1).
Relation between richness of native species (vertical axis) and cover of Lupinus polyphyllus (horizontal axis) in all of the three ranges (USA, Czech Republic, New Zealand). Species richness is expressed as ratios between the richness in a particular plot and maximum richness recorded in the sampled invaded plots in that particular range. Richness of native species on New Zealand reveals a negative relation to the cover of the alien Lupinus polyphyllus (full line, y = 0.783 - 0.074x, R2 = 0.573) with the most invaded plots (90% of cover of Lupinus polyphyllus, n = 7) harbouring on average only 4.8% of native species richness found in the most diverse plot sampled on New Zealand. Native species in the Czech Republic revealed negative relation to the cover of the alien Lupinus polyphyllus (dashed line, y = 0.644 - 0.017x, R2 = 0.087), but the relation was only marginally significant (p = 0.065, t = -1.8972, cor = -0.294, df = 1/38). In the native range of Lupinus polyphyllus s (USA), richness of native species revealed no relation to the cover of Lupinus polyphyllus (dotted line).
Response of species richness to the cover of dominant. Only species native to New Zealand revealed a significantly negative response to the cover of the alien Lupinus polyphyllus. The response of native species in Europe was negative, but only marginally significant.
| Predictor | Response variable | Correlation coefficient | P – value |
| cover of Lupinus polyphyllus | native species in its native range (NW USA) | 0.308 | 0.163 |
| cover of Lupinus polyphyllus | native species in the Czech Republic (central Europe) | -0.294 | 0.065 |
| cover of Lupinus polyphyllus | native species in New Zealand | -0.757 | < 0.001 |
| cover of Lupinus polyphyllus | alien species (of European origin) in New Zealand | -0.160 | 0.324 |
| cover of Hieracium pilosella agg. | native species in New Zealand | -0.104 | 0.523 |
| cover of Anthoxantum odoratum | native species in New Zealand | 0.070 | 0.666 |
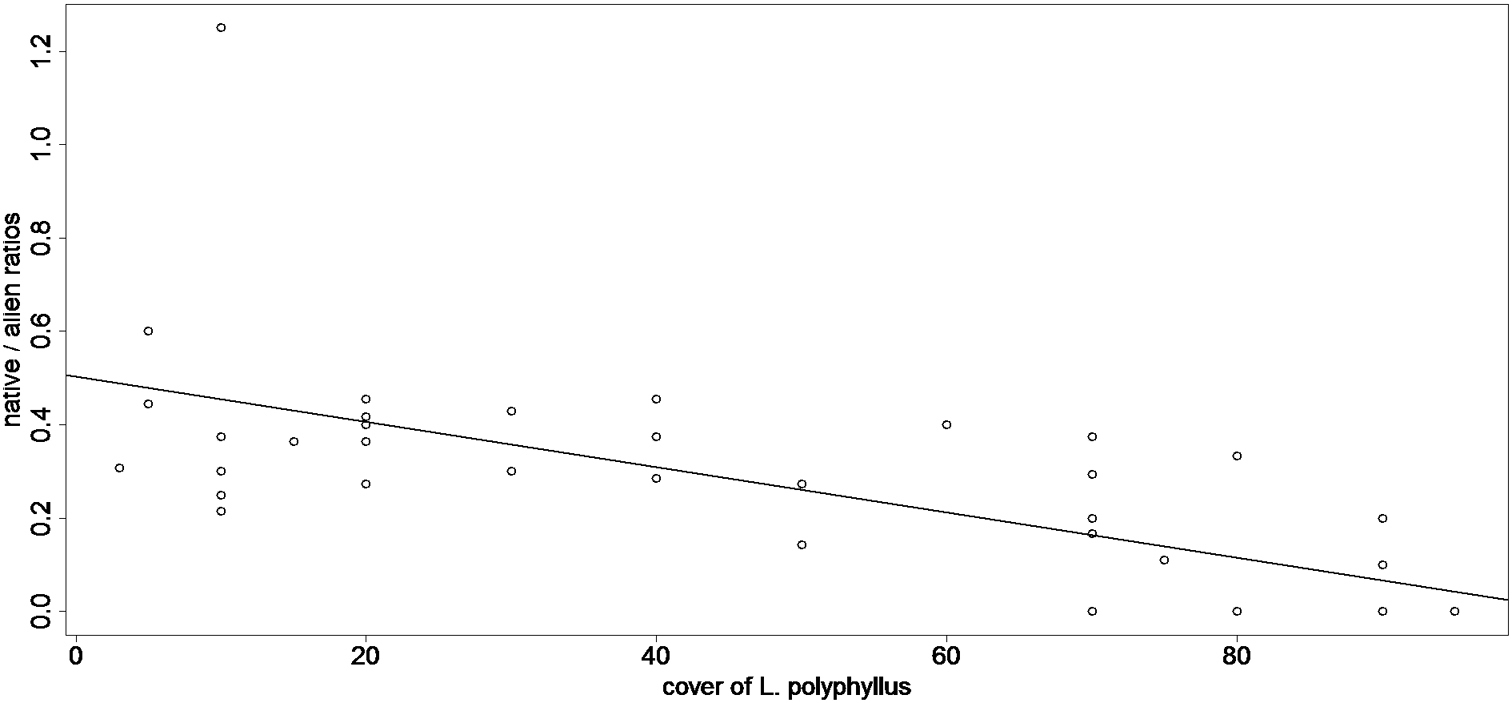
Relation between the ratios of native / alien species richness and the cover of Lupinus polyphyllus, as recored in New Zealand. A negative response (y = 0.503 - 0.049x, R2 = 0.445) shows that native species were much less successful in the stands with high cover of Lupinus polyphyllus compared to aliens, all of which were of European origin.
The response of species richness to the cover of Lupinus polyphyllus differed between the native and invaded ranges (p < 0.001, Chi = 32.15, DF = 2 / 94). In New Zealand, the ratios between the native / alien (of european origin) species richness responded to the cover of Lupinus polyphyllus negativelly (p = < 0.001, Chi = 23.547, DF = 1 / 36), indicating that alien species were more successful in the heavily invaded stands compared to native species.
The cover of Lupinus polyphyllus proved to be a significant predictor of species composition in all ranges (USA: p = 0.0220; Czech Republic: p = 0.0460; New Zealand: p = 0.0200 – Table 2).
In the USA (native range – Fig. 3) and Czech Republic (Fig. 4), some native species revealed negative response to the cover of Lupinus polyphyllus, while others were more abundant in plots with high cover of it. Contrary to this, all species native to New Zealand (Fig. 5) revealed negative response to the cover of the alien Lupinus polyphyllus, with the exception of Muehlenbeckia axillaris, which actually revealed a slight preference for the invaded stands.
The cover of Lupinus polyphyllus as a predictor of species composition. The table shows results of ordination models, where the cover of Lupinus polyphyllus was a predictor variable, while abundances of recorded species were the response variables. In all three ranges, the cover of Lupinus polyphyllus was found out to be a significant predictor of species composition – communities with low cover of Lupinus polyphyllus qualitatively differed from those with large covers of Lupinus polyphyllus.
| Range | F-ratio | p-value | Trace |
|---|---|---|---|
| Native range (USA) | 1.462 | 0.022 | 0.293 |
| Invaded range (New Zealand) | 1.597 | 0.02 | 0.21 |
| Invaded range (Czech Republic) | 1.404 | 0.046 | 0.18 |
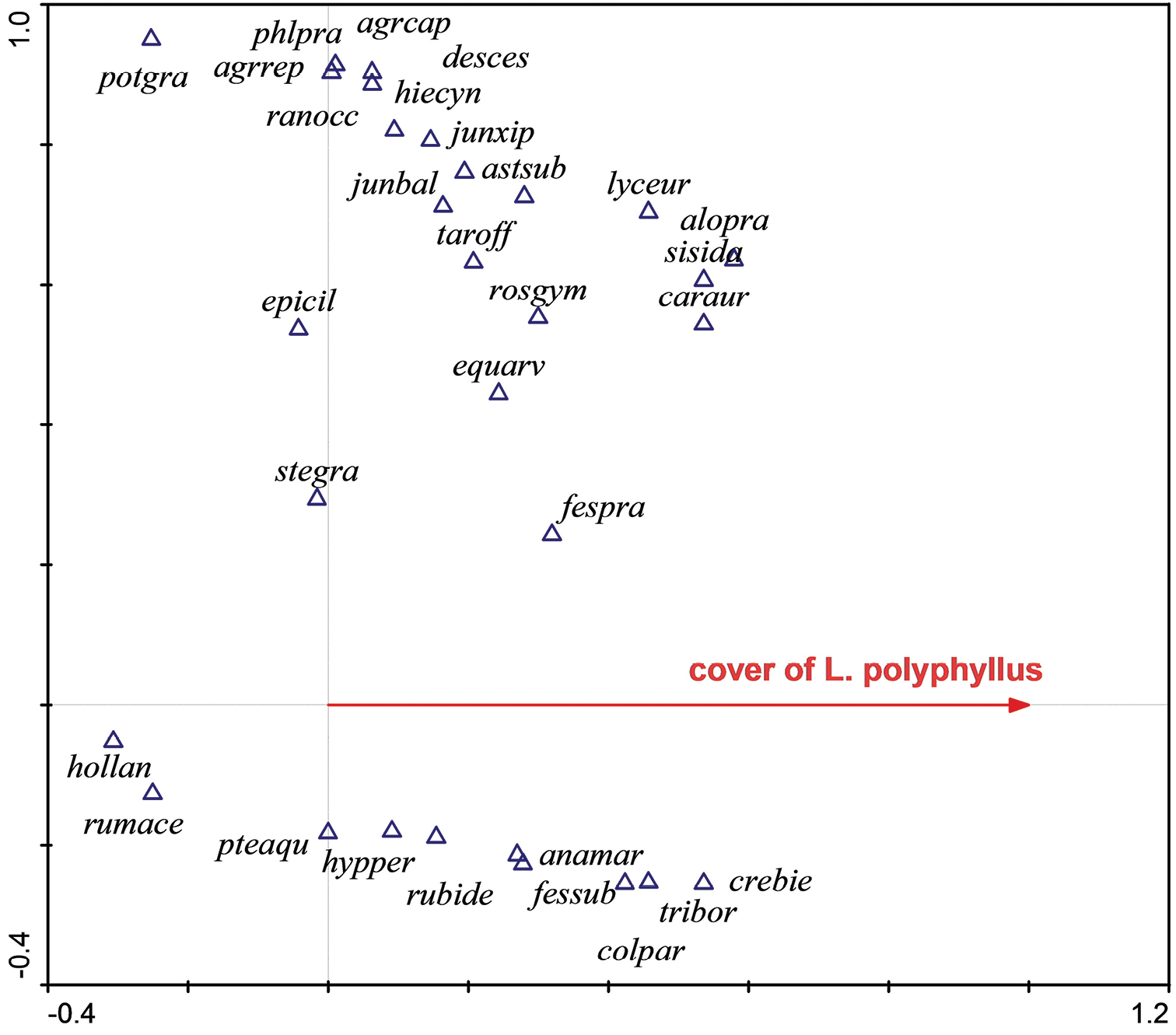
Ordination diagram (CCA) showing the response of species composition to the cover of Lupinus polyphyllus in its native range, USA. The first ordination axis (constrained or canonical axis) represents the predictor variable – the cover of Lupinus polyphyllus. Native species like Potentilla gracilis or Epilobium ciliatum reveal negative response to the cover of Lupinus polyphyllus, while others, like Anaphallis margaritacea, reveal a positive response. The second axis is unconstrained and represents some other environmental gradient, which is difficult to interpret in this case.
agrcap = Agrostis capillaris, agrrep = Agropyron repens, alopra = Alopecurus pratensis, anamar = Anaphallis margaritacea, astsub = Aster subspicatus, caraur = Carex aurea, colpar = Collinsia parviflora, crebie = Crepis biennis, desces = Deschampsia cespitosa, epicil = Epilobium ciliatum, equarv = Equisetum arvense, fespra = Festuca pratensis, hiecyn = Hieracium cynoglossoides, hollan = Holcus lanatus, hypper = Hypericum perforatum, junbal = Juncus balticus, junxip = Juncus xiphioides, lyceur = Lycopus europaeus, phlpra = Phleum pratense, potgra = Potentilla gracilis, pteaqu = Pteridium aquilinum, rosgym = Rosa gymnocarpa, rumace = Rumex acetosa, rubide = Rubus idaeus, stegra = Stellaria graminea, Sisyrrhynchium idahoense, taroff = Taraxacum officinale, tribor = Trientalis borealis
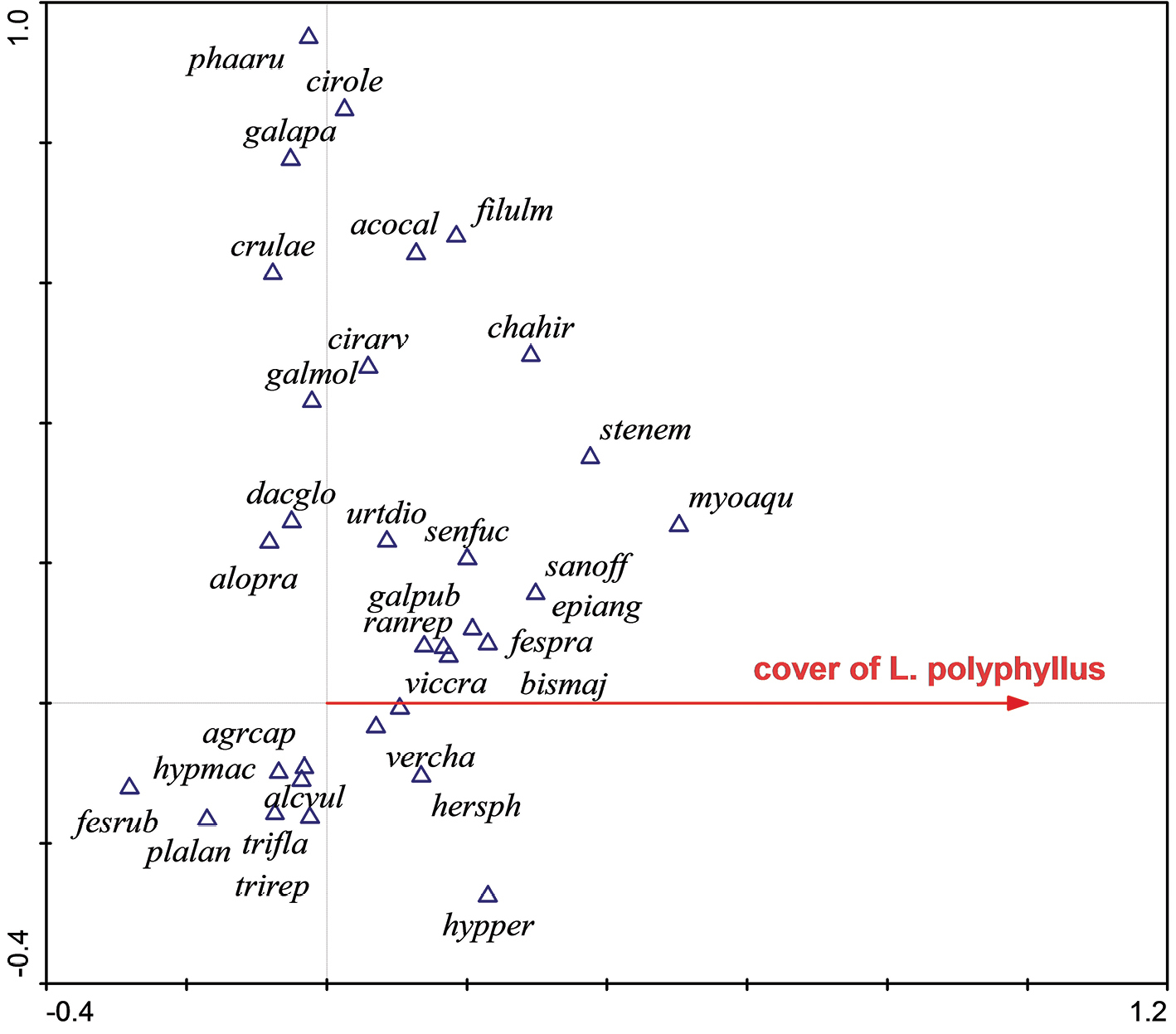
Ordination diagram (CCA) showing the response of species composition to the cover of Lupinus polyphyllus in the Czech Republic. The first ordination axis (constrained or canonical axis) represents the predictor variable – the cover of Lupinus polyphyllus. Same as in its native range, USA, some native species (Myosoton aquaticum, Stellaria nemorum, Bistorta major) reveal a positive relation to the cover of Lupinus polyphyllus, while others (Festuca rubra) reveal a strongly negative relation. The second axis is unconstrained and very likely represents some environmental gradient related to moisture, with species like Hypericum perforatum, Festuca rubra and Plantago lanceolata in the lower part and Phalaris arundinacea and Cirsium oleraceum in the upper part of the diagram.
acocal = Aconitum callibotryon, agrcap = Agrostis capillaris, alcvul = Alchemilla vulgaris agg., alopra= Alopecurus pratensis, bismaj= Bistorta major, chahir= Chaerophyllum hirsutum, cirarv = Cirsium arvense, cirole = Cirsium oleraceum, crulae= Cruciata laevipes, dacglo = Dactylis glomerata, epiang = Epilobium angustifolium, fespra = Festuca pratensis, fesrub = Festuca rubra, filulm = Filipendula ulmaria, galapa = Galium aparine, galmol = Galium mollugo, galpub = Galeopsis pubescens, hersph = Heracleum sphondilium, hypmac = Hypericum maculatum, hypper = hypericum perforatum, myoaqu = Myosoton aquaticum, phaaru = Phalaris arundinacea, plalan = plantago lanceolata, ranrep = Ranucnulus repens, sanoff = Sanguisorba officinalis, senfuc = Senecio fuchsi agg., stenem = Stellaria nemorum, trifla = Trisetum flavescens, trirep = Trifolium repens, urtdio = Urtica dioica, vercha = Veronica chamaedrys, viccra = Vicia cracca
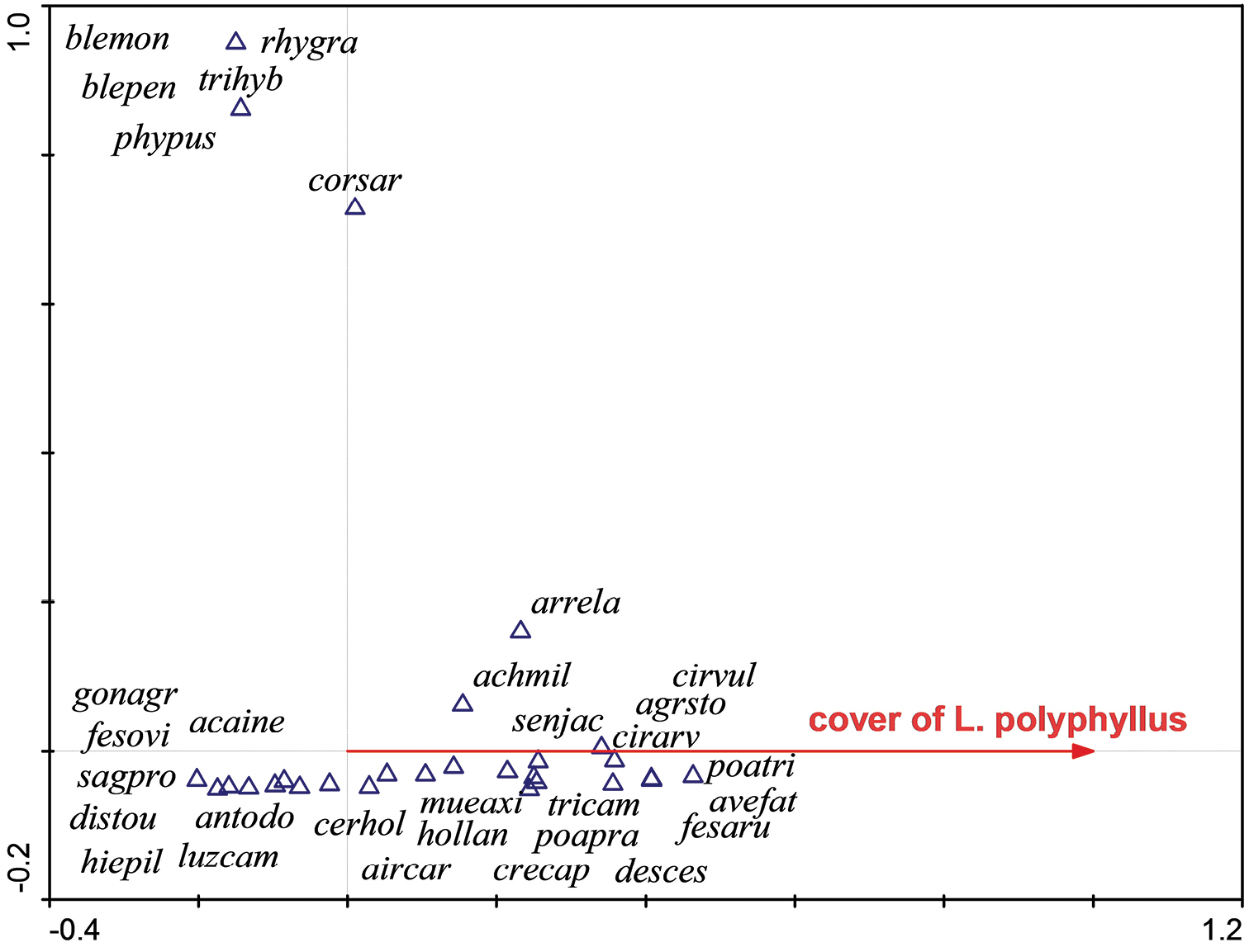
Ordination diagram (CCA) showing the response of species composition to the cover of Lupinus polyphyllus in New Zealand. The first ordination axis is constrained and represents the predictor variable – the cover of Lupinus polyphyllus.
All species native to New Zealand revealed a negative relation (Discaria toumatou, Acaena inermis, Gonocarpus aggregatus, Rhytidosperma gracilis), with the exception of Muehlenbeckia axillaris, which reveals a positive relation to the cover of Lupinus polyphyllus. The second ordination axis (vertical) is unconstrained and difficult to interpret in this case. acaine = Acaena inermis, achmil = Achillea millefolium agg., agrsto = Agrostis stolonifera, aircar = Aira caryophyllea, antodo = Anthoxantum odoratum, arreal = Arrhenatherum elatius, avefat = Avenula fatua, blemon = Blechnum montanum, blepen = Blechnum penna – marina, cerhol = Cerastium holosteoides, cirarv = Cirsium arvense, cirvul = Cirsium vulgare, corsar = Coriaria sarmentosa, crecap = - Crepis capillaris, desces = Deschampsia cespitosa, distou = Discaria toumatou, fesaru = Festuca arundinacea, fesovi = Festuca ovina, gonocr = Gonocarpus aggregatus, hiepil = Hieracium pilosella agg., hollan = Holcus lanatus, luzcam = Luzula campestris, mueaxi = Muehlenbeckia axillaris, phypus = Phymatosorus pustulatus, poapra = Poa pratensis, poatri = Poa trivialis, rhygra = Rhytidosperma gracilis, sagpro = Sagina procumbens, senjac = Senecio jacobaea, tricam = Trifolium campestre, trihyb = Trifolium hybridum
All these results need to be interpreted with caution, mainly given by the comparative way the data were sampled. It is possible that the factor of the alien´s dominance is confounded with other environmental factors, such as anthropogenic disturbance or increased nutrient levels, which may suppress native species and enhance the alien´s dominance. It is not really possible to say if the invasion (expressed as the alien´s dominance on a given small spatial scale in this study) is promoted by these changes, or if the alien species transforms the sites actively. Alien invasive species have been documented to change site conditions massively, mainly due to substrate stabilisation (Lupinus polyphyllus –
Be that as it may, the results show that in the invaded ranges, the dominance of Lupinus polyphyllus is associated with site conditions that do not favor native species, which is especially apparent in New Zealand. Contrary to that, species from the native range were successful when growing with Lupinus polyphyllus, more than species from both invaded ranges (Czech Republic and New Zealand). This is likely to be due to these species´ being well apapted to the conditions of sites distinctively dominated by Lupinus polyphyllus, which may be caused by the long-term coevolution of communities and species´ filtering in the dominant species´ native range. Due to the long-term presence of the dominant species Lupinus polyphyllus, species in the native range have been selected to coexist with it, otherwise they would have been eliminated from communities where Lupinus polyphyllus is a dominant species.
Contrary to that, Lupinus polyphyllus is a newly imported dominant species to the communities in the invaded ranges. In Europe, the invasion of Lupinus polyphyllus is associated with some loss of native species richness (see for example
Species native to New Zealand were found to be least successful when growing in the stands of Lupinus polyphyllus in this study, with the most invaded plots (with the cover of Lupinus polyphyllus of 90%) being almost free of species native to New Zealand. This can be partly related to the fact that, in New Zealand, Lupinus polyphyllus often invades relatively unstable and regularly disturbed riparian terraces where the vegetation is not really dense, so the level of interspecific competition can be expected to be low. Therefore, heliophilous species of these communities (Coriaria plumosa, Epilobium melanocaulon, Parahebe decora, Raoullia subsericera, Raoullia hookeri, Wahlenbergia albomarginata) are weak competitors when confronted with the distinctive alien dominant and this can be caused mainly by the differences in the type of invaded habitats between these two distinct invaded ranges (Czech Republic and New Zealand). On the other hand, Lupinus polyphyllus also invades more stable and less frequently disturbed riparian meadows in New Zealand, with species like Acaena inermis, Carex geminata, Gonocarpus aggregatus or Prasophyllum colensoi on wet places and Brachyglottis bellidioides, Celmisia gracillenta, Discaria toumatou or Leucopogon fraseri on dryer sites. In these communities, the vegetation is dense and the level of interspecific competition can be expected to be rather high, but native species still fail to coexist with the dominant invasive alien Lupinus polyphyllus. It is possible that the intensity of interspecific competition is generally lower in the communities of New Zealand and native species are weaker competitors due to, for example, the effects of insularity, which means a long-term isolated development and not having been confronted with competitively strong species with cosmopolitan tendencies. At the same time, the insular flora of New Zealand can be expected to be phylogenetically rather homogenous, originating from a few clades originally colonizing the islands. Such communities have been documented to be more easily invaded (
In New Zealand, Lupinus polyphyllus has been documented to accumulate silt material and therefore accelerate the stabilization of riparian terraces (
The target communities in New Zealand were also heavilly invaded by other aliens, like Anthoxantum odoratum and Hieracium pilosella agg., so the potential impact of the invasive alien Lupinus polyphyllus was heavily confounded with possible impacts of other invasive species. Anthoxantum odoratum forms dense and homogenous stands, while Hieracium pilosella agg. forms dense 'pillows' of leaf rosettes. But richness of species native to New Zealand was not found to be related to the cover of either of these aliens of european origin, when sampled and tested in the same way as the relation to the cover of Lupinus polyphyllus. Some native species, like Brachyglottis bellidioides, Coprosma atropurpurea or Celmisia gracillenta were actually found to prefer places with large covers of Hieracium pilosella´s rosettes. For these reasons, it is highly likely that the site conditions that depauperate the diversity of communities in New Zealand are associated with the invasion by Lupinus polyphyllus, rather than with other abundant aliens, such as Anthoxantum odoratum and Hieracium pilosella agg.
The data shows that species in the native range are able to coexist with the dominant lupins better than species from the invaded ranges. Out of the two invaded ranges studied, species native to New Zealand were found to be most effectively eliminated from communities dominated by the alien Lupinus polyphyllus. An uncertainty remains whether this effect is caused by the invading Lupinus polyphyllus or by other environmental factors that promote the invasion, such as human induced disturbance, nitrification or substrate stabilisation, however, some of these changes can be promoted by the invasion by Lupinus polyphyllus too. Even though it is difficult to separate causes and effects of the invasion in this case (as it is with most invasions), the results show that different native species respond differently to the invasion by a single alien. In one invaded range – Europe, most species are able to coexist with the invasive Lupinus polyphyllus, while in New Zealand, native species are virtually eliminated from stands with a high cover of the dominant alien Lupinus polyphyllus. It remains questionable to which degree the results scale up from tiny vegetation plots to larger units in New Zealand. The results show that at the fine scale, the invasion is associated with a severe degradation of communities, so it is likely that its potential impacts are apparent even at larger scales, due to, for example, reduction of populations of native species. In the extreme cases, this can lead to local extinctions in areas with large stands of Lupinus polyphyllus, such as the valley of Waimakariri river in the Arthur Pass National Park, NZ. Moreover, this alien invades pristine areas with many rare species and its invasion therefore represents a serious threat to native plant diversity at the fine scale and a threat to landscape character at larger scales. High local abundances observed in the invaded ranges suggest that Lupinus polyphyllus has the potential to spread further, well beyond the boundaries of its current distribution.
The work on this manuscript was funded by grants no. P505/11/1112 and 206/070668 from the Grant Agency of the Czech Republic, an International Collaboration Support no. M200050971 from the Czech Academy of Sciences and by an institutional long-term research plan no. AV0Z60050516. A great thanks goes to Petr Pyšek for supervising the project and helping me find my research focus and to my wife Hana Hejdová for being generous and forgiving. Another great thanks goes to my uncle Joe Mullen for careful language revision of the manuscript and to my colleague Honza Pergl for his assisstance with processing the figures.
Entry data for the univariate models with species richness as a response variable. (doi: 10.3897/neobiota.17.4317.app1) File format: Micrisoft Excell document (xls).
Explanation note: The file presents the entry data for i) the mixed effect model testing the differences between all three ranges and ii) data with native / alien species richness ratios used for testing the response of native species versus aliens of european origin on New Zealand.
Raw data on species composition and abundances, expressed as the percentage covers of recorded species. (doi: 10.3897/neobiota.17.4317.app2) File format: Micrisoft Excell document (xls).
Explanation note: The data with species composition were used for the multivariate ordination models, testing the response of individual species to the gradient of the cover of Lupinus polyphyllus.