| Taxon names | Citations | Turn highlighting On/Off |







(C) 2013 Enelge Gildenhuys. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Citation: Gildenhuys E, Ellis AG, Carroll SP, Le Roux JJ (2013) The ecology, biogeography, history and future of two globally important weeds: Cardiospermum halicacabum Linn. and C. grandiflorum Sw. NeoBiota 19: 45–65. doi: 10.3897/neobiota.19.5279
Members of the balloon vine genus, Cardiospermum, have been extensively moved around the globe as medicinal and horticultural species, two of which are now widespread invasive species; Cardiospermum grandiflorum and Cardiospermum halicacabum. A third species, Cardiospermum corindum, may also have significant invasion potential. However, in some regions the native status of these species is not clear, hampering management. For example, in South Africa it is unknown whether Cardiospermum halicacabum and Cardiospermum corindum are native, and this is a major constraint to on-going biological control programmes against invasive Cardiospermum grandiflorum. We review the geography, biology and ecology of selected members of the genus with an emphasis on the two most widespread invaders, Cardiospermum halicacabum and Cardiospermum grandiflorum. Specifically, we use molecular data to reconstruct a phylogeny of the group in order to shed light on the native ranges of Cardiospermum halicacabum and Cardiospermum corindum in southern Africa. Phylogenetic analyses indicate that southern African accessions of these species are closely related to South American taxa indicating human-mediated introduction and/or natural long distance dispersal. Then, on a global scale we use species distribution modelling to predict potential suitable climate regions where these species are currently absent. Native range data were used to test the accuracy with which bioclimatic modelling can identify the known invasive ranges of these species. Results show that Cardiospermum species have potential to spread further in already invaded or introduced regions in Australia, Africa and Asia, underlining the importance of resolving taxonomic uncertainties for future management efforts. Bioclimatic modelling predicts Australia to have highly favourable environmental conditions for Cardiospermum corindum and therefore vigilance against this species should be high. Species distribution modelling showed that native range data over fit predicted suitable ranges, and that factors other than climate influence establishment potential. This review opens the door to better understand the global biogeography of the genus Cardiospermum, with direct implications for management, while also highlighting gaps in current research.
Balloon vines, biological invasion, C. corindum, management, phylogeny, species distribution modelling
Understanding the biology, ecological requirements, and native distributions of potentially invasive species is crucial to ensure effective management and to predict their potential invasiveness. We review these attributes for selected members of a globally weedy genus, Cardiospermum, commonly known as balloon vines. We review the ecology and history of anthropogenic range expansion of the genus, with special emphasis on the two most problematic species in the group, Cardiospermum grandiflorum and Cardiospermum halicacabum. On a regional scale we aim to resolve the native provenance(s) of balloon vine species found in southern Africa, using a phylogenetic approach. Lastly, on a broad scale we assess the invasion risk posed by balloon vine species found outside their supposed native ranges, using species distribution modelling. Moreover, to evaluate the merit of this commonly employed method, we compare data of known invaded areas to predictions based on native range records.
The genus Cardiospermum L. 1753 (family Sapindaceae, tribe Paullinieae) currently consists of 17 shrub, subshrub, climber, and erect species, commonly called balloon vines (
Morphology divides this genus into three sections; Cardiospermum Radlk., Carphospermum Radlk. and Ceratadenia Radlk. (
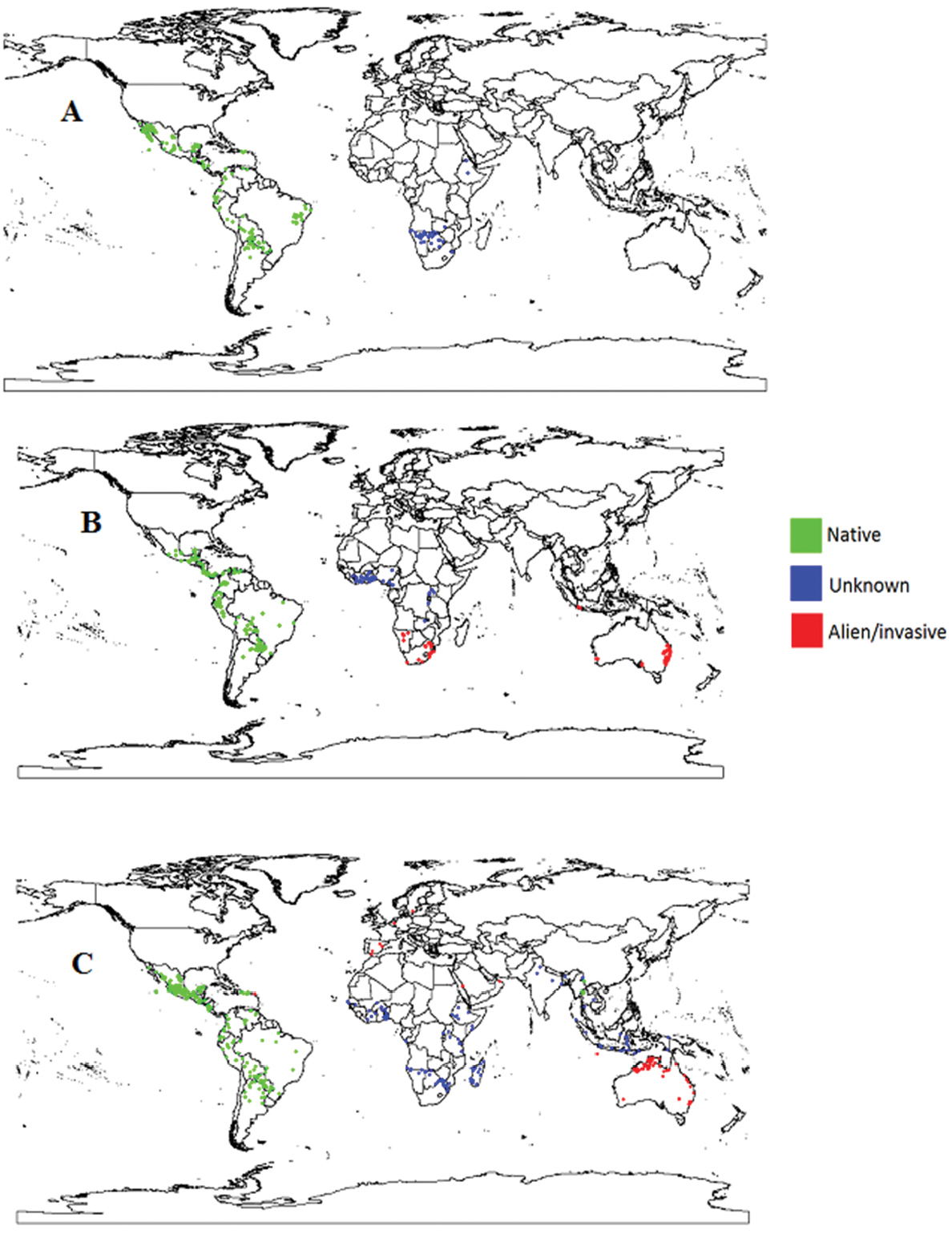
Only four Cardiospermum species occur abundantly outside the neotropics: Cardiospermum halicacabum, Cardiospermum grandiflorum, Cardiospermum corindum, and Cardiospermum pechuelii (
Distribution of Cardiospermum species. Global distribution of A Cardiospermum corindum B Cardiospermum grandiflorum and C Cardiospermum halicacabum in native, unknown and alien or invasive regions.
Details of uncertain native or non-native statuses of two Cardiospermum species in North America and Africa.
| Continent | References for debated native/non-native status | |
|---|---|---|
| Cardiospermum halicacabum | North America | |
| Africa | ||
| Cardiospermum corindum | Africa | |
| North America |
Alien invasive species are a global concern and a threat to biodiversity (
The ornamental attraction of Cardiospermum species are their inflated balloon shaped fruit (Fig. 2). Coincidently this trait also contributes to their colonisation success, since these balloons can float in seawater and stay viable for long periods of time, facilitating long distance dispersal, even between landmasses (
Cardiospermum fruit. The ornamental attraction of Cardiospermum plants and the reason for their widespread distribution is their balloon shaped fruit A Cardiospermum grandiflorum (E Gildenhuys) and B Cardiospermum halicacabum (J-Y Meyer).
Invasive Cardiospermum species are considered “transformer weeds” (
Currently two Cardiospermum species are globally considered important invaders. Cardiospermum grandiflorum is classified as an invasive species in Australia, southern Africa, Cook Islands and many other Pacific islands (
South Africa’s Working for Water program launched a research initiative in 2003 to find biological control agents against Cardiospermum grandiflorum (
The ornamental trade of Cardiospermum halicacabum and Cardiospermum grandiflorum spans more than 100 years. For example, in Australia the first herbarium records of Cardiospermum grandiflorum date back to 1923, collected around Sydney, New South Wales (
The introduction of Cardiospermum grandiflorum into South Africa occurred approximately 100 years ago (
Cardiospermum halicacabum and Cardiospermum grandiflorum are also present in North America (
Cardiospermum halicacabum is also present in China and India. In China it is described as a common weed in forest margins, shrublands, grasslands, cultivated areas and wastelands of the east, south and southwest (Flora of China, www.eFloras.org) – though considered native by some – [Pacific Island Ecosystems at Risk (PIER)]. In India it is widespread and considered non-native (
A comprehensive understanding of the biology and ecology of Cardiospermum halicacabum and Cardiospermum grandiflorum is important because of the invasive potential and biogeographic uncertainties which characterise these two taxa. Such information will also contribute to making informed decisions on their conservation (if native) or control (if invasive). This is especially true since the extent to which thesespecies are invasive is essentially unknown and the uncertainties of their classification in most areas suggest the possibility of a cosmopolitan native distribution.
The morphology of these two species is similar, with both being adapted for tropical and subtropical climates. Cardiospermum grandiflorum is a large, semi-woody perennial, whereas Cardiospermum halicacabum is smaller, less woody and commonly annual. Cardiospermum grandiflorum has elongated fruit (4.5–6.5 cm in length) compared to the more compact fruit of Cardiospermum halicacabum (2.5–3.0 cm in length) (Fig. 2A and B). Fruit structures consist of three dorsally keeled membranous capsules each consisting of three internal blades (
Both taxa produce flavone aglycones and cyanogenic compounds that likely protect them against predators such as soapberry bugs (
The germination and growth success of Cardiospermum halicacabum is well studied because of its medicinal value, as well as its impact on soybean plantations and on natural riparian areas (
Despite morphological similarity, these two species differ markedly. They occasionally occur sympatrically but mostly prefer different habitats with Cardiospermum halicacabum dominating tropical and Cardiospermum grandiflorum subtropical areas (
To date, managing and reducing impacts of Cardiospermum invasions has mostly involved manual removal or burning (
In collaboration with South Africa’s Working for Water program, a biological control programme was initiated against Cardiospermum grandiflorum in 2003. However due to the taxonomic uncertainty surrounding Cardiospermum halicacabum and Cardiospermum corindum (discussed earlier, Table 1), biocontrol agents cannot be released, hampering effective management in South Africa. The importance of clarifying the geographic native ranges of all Cardiospermum species currently found in South Africa for the successful biological control of Cardiospermum grandiflorum is therefore evident. If Cardiospermum corindum and Cardiospermum halicacabum are indeed native to southern Africa, only agents that are specific on Cardiospermum grandiflorum can qualify for release in South Africa, and thus far, these agents have proved particularly difficult to rear and test under quarantine conditions (D. Simelane, pers. comm.). On the other hand, if Cardiospermum corindum and Cardiospermum halicacabum are not native to southern Africa, all suitable agents against Cardiospermum grandiflorum qualify for release in South Africa.
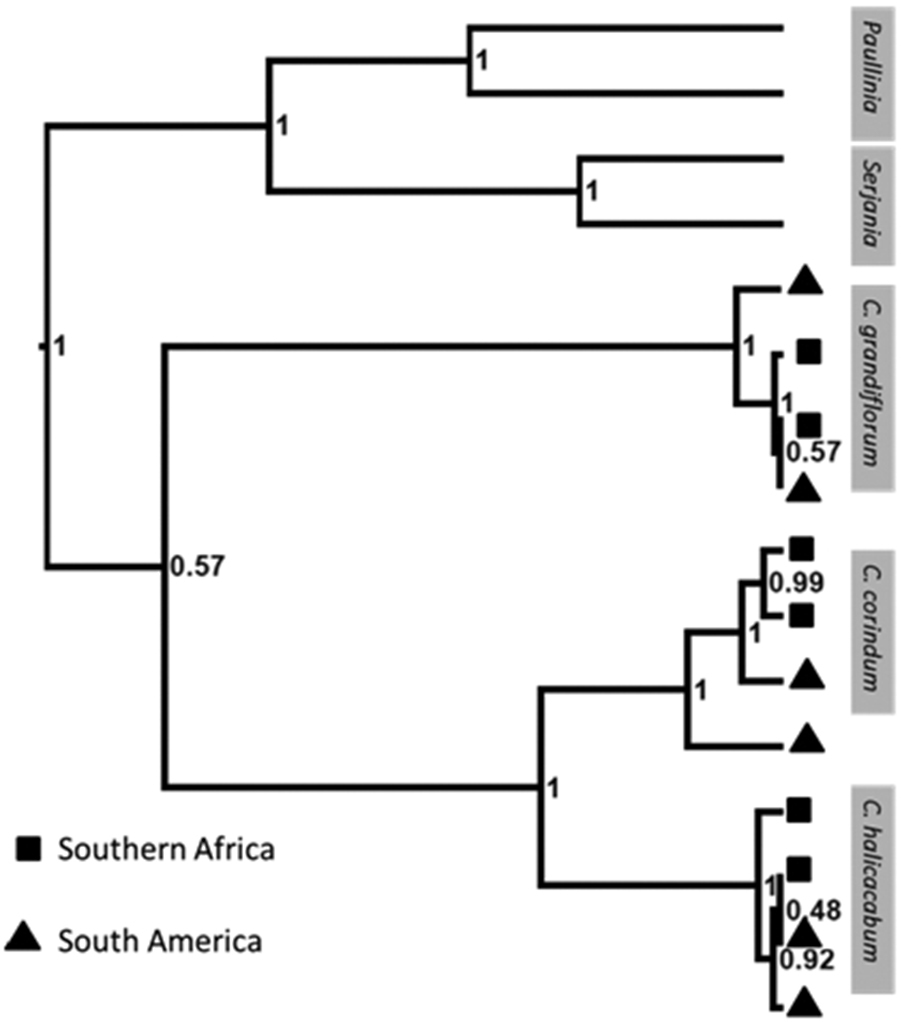
To determine the relationship between Cardiospermum species occurring in Africa and South America we sequenced two accessions of Cardiospermum grandiflorum, Cardiospermum halicacabum and Cardiospermum corindum from each continent (South America and Africa). DNA was extracted from dried plant material using the CTAB method (
The retrieved phylogeny indicates a close relationship between samples from South America and southern Africa (Fig. 3). For Cardiospermum grandiflorum and Cardiospermum halicacabum southern African samples are more closely related to South American samples than to other samples from southern Africa (i.e. geographic paraphyly). It is therefore likely that Cardiospermum halicacabum in southern Africa, like Cardiospermum grandiflorum, represents a recent introduction, and is therefore not native. For Cardiospermum corindum however the phylogeny cannot dismiss natural long distance dispersal as an explanation for the species’ presence in southern Africa, due to the southern African accessions forming a monophyletic group within the South American clade. The ability of Cardiospermum fruit to float in seawater for long periods of time and remain viable, makes a strong case for long distance dispersal. In order to clarify the uncertainty around human introduction versus rare long distance dispersal events, future phylogenetic analyses should include more and geographically widespread collections.
Cardiospermum phylogeny. Phylogeny of six South American and six southern African accessions of Cardiospermum species with Paullinia and Serjania species used as outgroup taxa. Topology support is shown as posterior probability at each node.
Prevention is better than cure, with eradication of introduced species typically becoming less feasible as spread progresses (
We used BIOMOD version 1.1.5 (
Contribution (%) of each BioClim variable used for distribution modelling of Cardiospermum species. The first value in each species column is for global and the second for native range modelling.
| Variables used for modelling | Variable importance | |||||
|---|---|---|---|---|---|---|
| Cardiospermum halicacabum | Cardiospermum grandiflorum | Cardiospermum corindum | ||||
| Global | Native | Global | Native | Global | Native | |
| Min temperature of the coldest month | 21.2 | 12.5 | 13.8 | 25.4 | 14.9 | 21.1 |
| Max temperature of the warmest month | 6.2 | 2.3 | 4 | 0.9 | 3.9 | 1.7 |
| Precipitation of the coldest quarter | 4.9 | 22.2 | 27.8 | 25.9 | 13.7 | 2.1 |
| Precipitation of the driest month | 2 | 1.1 | 13.1 | 2.5 | 3.6 | 16.9 |
| Precipitation of the warmest quarter | 44.5 | 8.3 | 20 | 22.1 | 31.5 | 2.7 |
| Temperature seasonality | 17.2 | 57.6 | 22.8 | 24.9 | 6 | 7.9 |
| Precipitation of the wettest quarter | - | - | 3.2 | 5.7 | 34 | 42.5 |
The accuracy of species distribution modelling is influenced by false positives and negatives (
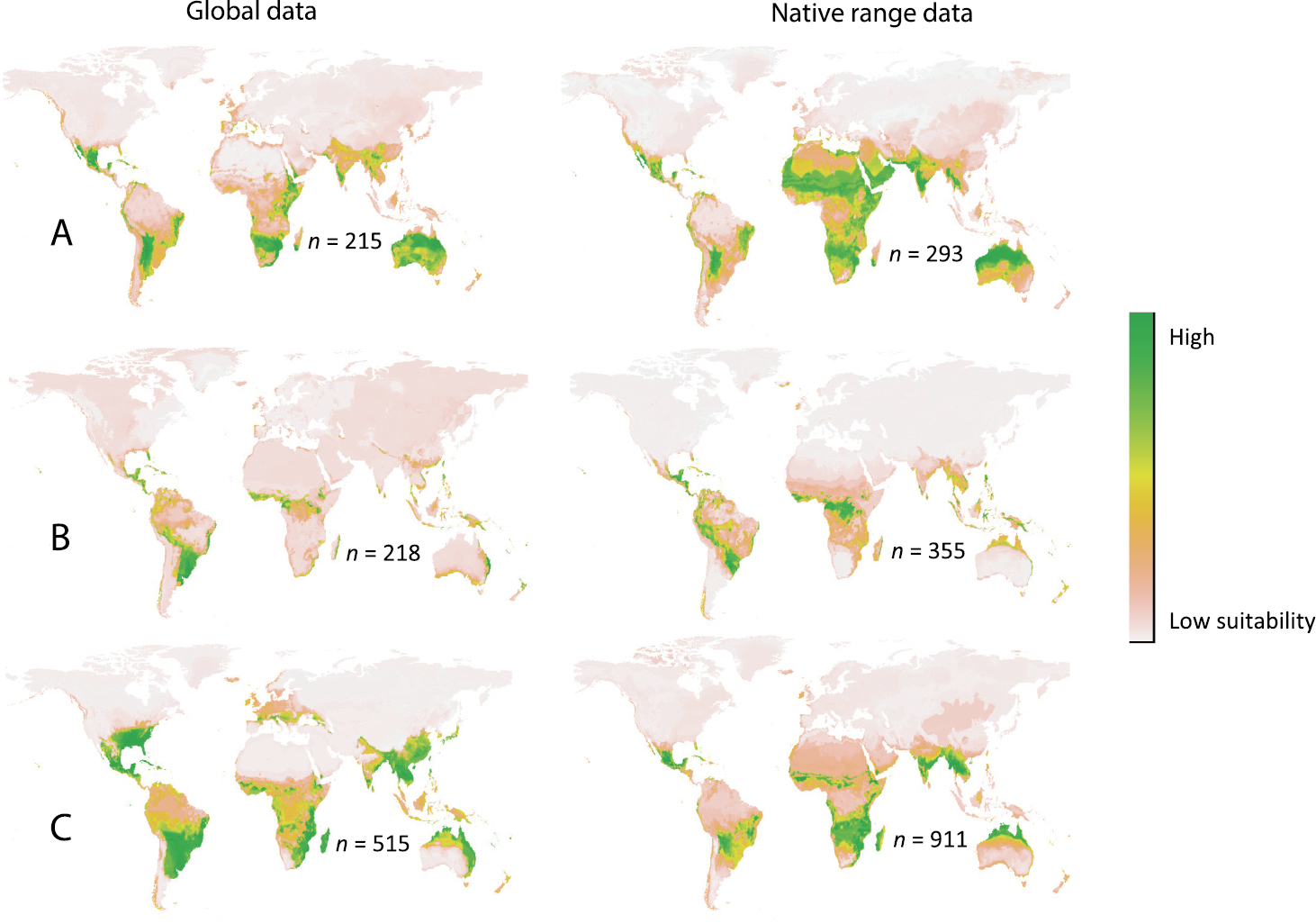
Australia: Global data models for all three species performed well, with AUC values above 0.9 and TSS values above 0.65 (Table 3). Bioclimatic predictions show that a large proportion of Australia is climatically suitable for Cardiospermum corindum, a species currently absent in this country. Both Cardiospermum halicacabum and Cardiospermum grandiflorum have been introduced to Australia and are classified as invasive weeds. The suitable climate range for Cardiospermum corindum in Australia is much larger than predicted for both Cardiospermum grandiflorum and Cardiospermum halicacabum and as such ornamental or medicinal introductions of Cardiospermum corindum into Australia should be prevented (Fig. 4A, B, C). Modelling also predicted that the east coast of Australia is climatically highly suitable for Cardiospermum halicacabum, such that any risks from its establishment in this area should be assessed. Cardiospermum grandiflorum appears to be a more rapid colonizer than Cardiospermum halicacabum in Australia and it is already present in most predicted areas. It is however likely to become locally more abundant in areas where it is already found (Fig. 1B and Fig. 4B).
Evaluation of modelling predictions. True skill statistic (TSS) and area under the receiver operating characteristic (ROC curve) (AUC) for global and native range modelling of three widespread Cardiospermum species. The first value in TSS and AUC column is for global and the second for native range modelling. Independent data evaluation is for the native range models evaluated against known non-native ranges.
| Species | TSS | Independent data (TSS) | AUC | Independent data (AUC) | ||
|---|---|---|---|---|---|---|
| Global | Native | Native | Global | Native | Native | |
| Cardiospermum halicacabum | 0.651 | 0.703 | 0.441 | 0.9 | 0.923 | 0.755 |
| Cardiospermum grandiflorum | 0.759 | 0.665 | 0.343 | 0.95 | 0.895 | 0.639 |
| Cardiospermum corindum | 0.689 | 0.629 | 0.565 | 0.905 | 0.896 | 0.881 |
Species distribution modelling of Cardiospermum species. Global climatically suitable ranges for A Cardiospermum corindum B Cardiospermum grandiflorum, and C Cardiospermum halicacabum as predicted by boosted regression trees in BIOMOD using global (left) and native range data (right). Number of occurrence points used for modelling (n) is indicated on each map.
Europe and Asia: Our modelling approach identified Europe as mostly climatically unsuitable for Cardiospermum (Fig. 4A, B, C). Areas of suitable climate are present for all three species in certain parts of Asia including India (where Cardiospermum halicacabum and Cardiospermum corindum are present), Thailand and Pakistan, with Cardiospermum grandiflorum potentially being the most restricted taxon (Fig. 4B). Cardiospermum corindum has high climatic suitability in southern Yemen, southern India, Thailand, Myanmar and southern China (Fig. 4A). The southernmost tip of Yemen seems climatically suitable for Cardiospermum halicacabum, with India, Thailand, Cambodia, Vietnam, Myanmar, Japan, Taiwan and parts of China highly suitable (Fig. 4C). Many of these regions are already occupied by Cardiospermum halicacabum. Climatically suitable habitat for Cardiospermum grandiflorum in Asia only appears to be present in southern India, Sri Lanka and parts of Vietnam (Fig. 4B).
Southern Africa: In South Africa bioclimatically suitable areas for Cardiospermum grandiflorum are in the Western Cape Province, while for Cardiospermum halicacabum they are in coastal areas in the Eastern Cape Province. Bioclimatically suitable areas in South Africa are the largest for Cardiospermum corindum, with the Western and Eastern Cape Provinces being highly suitable. Currently the species is limited to Limpopo, Mpumalanga and northern parts of Kwazulu Natal (SANBI). Spread and anthropogenic movements of Cardiospermum species in South Africa should therefore be closely monitored since a large part of South Africa appears climatically suitable for establishment. While Cardiospermum grandiflorum and Cardiospermum halicacabum are recorded as naturalised in parts of Namibia and Botswana, bioclimatic modelling did not predict either country as climatically suitable. Cardiospermum species are not widespread in these two countries and possibly only occur in areas with suitable microclimates. Such habitats typically differ significantly from surrounding environments and often result from human actions, and are therefore excluded in bioclimatic modelling based on more coarse data, such as this study (
Models calibrated with South and Central American native occurrence records performed fairly well when cross-validated using AUC and TSS, with values higher than 0.85 and 0.6 respectively. However this was not the case when these models were evaluated with independent data, thus known presence data not used in modelling. Cardiospermum halicacabum and Cardiospermum grandiflorum had low AUC and TSS values ranging between 0.60–0.80 and 0.30–0.45 respectively, only Cardiospermum corindum models performed fairly well (AUC > 0.85 and TSS > 0.55, Table 3).
These results indicate that models calibrated with native range occurrence records only, would not have accurately predicted the invasive spread of Cardiospermum grandiflorum in South Africa while underestimating its potential range in Australia. This lack of accuracy for identifying invasive regions using native data questions the suitability of using species distribution modelling alone when determining potential invasive regions.
Also contrary to what we expected, models calibrated using native range data predicted larger climatically suitable areas than models calibrated with global range data (Fig. 4; except for Cardiospermum halicacabum). We hypothesised that this is due to the more restricted climate zones created with the widespread pseudo-absence data of the global range, thus including more diverse habitats to exclude as suitable areas. We plotted the presence and absence points for both native and global range data for each variable against the probability of occurrence using the response plot function in R (Appendix, Fig. S1 A–F). In these figures it is clear that global data variables include a wider environmental range for pseudo-absences compared to the native range pseudo-absences, especially when considering the most significant variables based on variable importance (Table 2). To test if this is indeed the case we ran three additional models with the same settings as the previous models but using native range presence data and global pseudo-absences data. We used the same evaluation parameters as for the previous models (Appendix, Table S1, S2). This approach resulted in projections that more closely resembled global range model predictions or are even more restricted predictions (Appendix, Fig. S2). These results indicate that while native range data can be used to predict potential suitable areas, data are often over-fitted, thus over predict the extent of suitable habitats, due to less restricted absence data created from the native range.
While species distribution modelling is a popular tool for predicting potential invasive ranges its accuracy remains questionable (
Thus, taking the contradicting results into account and also considering the many other factors that influences a species distributional range, lead us to conclude that while bioclimatic modelling is a useful approach, it should not be used as a stand-alone tool when making conservation decisions regarding the introduction of species into a novel range and caution should be exercised to ensure the quality of input data while also taking other factors into account as discussed above.
Many regions globally appear climatically suitable for establishment of Cardiospermum grandiflorum, Cardiospermum corindum and Cardiospermum halicacabum, cautioning against further introductions. Resolving the native ranges for these species globally is therefore important for biodiversity conservation and invasive species management. For example, our preliminary results indicate that Cardiospermum halicacabum from southern Africa have a close relationship with South American samples, but that rare long distance dispersal cannot be ruled out as an explanation, while the split between South American and southern African Cardiospermum corindum hints towards a native status on both continents. Future work should include a more comprehensive phylogeny to substantiate our findings, including balloon vine specimens from other biogeographic regions where the native status is known. If it is found that they are indeed alien to Africa and Asia, a risk assessment challenge lies ahead since large areas of these continents appear climatically suitable for their establishment. No Cardiospermum species are regarded as native in Australia, and measures to limit the spread of Cardiospermum halicacabum and Cardiospermum grandiflorum may be augmented with biological control measures that include native soapberry bugs that are evolving to use them more efficiently (
Cardiospermum species are also used by many people in rural areas for medicinal purposes, further emphasizing a need to resolve the natal biogeographic distribution of this globally important genus to ensure its effective management, control or conservation.
We thank Dr Ingolf Kühn and the two anonymous reviewers for their constructive comments on previous drafts of the manuscript. Financial support was provided by the DST-NRF Centre of Excellence for Invasion Biology and the Working for Water Programme through their collaborative project on “Research for Integrated Management of Invasive Alien Species”. E Gildenhuys acknowledges the South African National Research Foundation’s (NRF) Scarce Skills scholarship programme. J Le Roux also acknowledges Stellenbosch University’s Sub-committee B “Young Researchers Fund” and the NRF Thuthuka Programme for research funding. S Carroll acknowledges support from the School of Life Sciences at the University of Queensland, St. Lucia. We are grateful to Jason Donaldson and Vernon Visser for their help and advice with species distribution modelling.
Supporting information for species distribution modelling of Cardiospermum species using native range presences and global pseudo absences. (doi: 10.3897/neobiota.19.5279.app) File format: Micrisoft Word Document (doc).
Explanation note: The file contains the response plots for variables used in species distribution modelling. Modelling predictions and the importance of individual variables in those models using native range presence and global absence data are also given.