(C) 2014 Graham D. Bonnett. This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Citation: Bonnett GD, Kushner JNS, Saltonstall K (2014) The reproductive biology of Saccharum spontaneum L.: implications for management of this invasive weed in Panama. NeoBiota 20: 61–79. doi: 10.3897/neobiota.20.6163
Saccharum spontaneum L. is an invasive grass that has spread extensively in disturbed areas throughout the Panama Canal watershed (PCW), where it has created a fire hazard and inhibited reforestation efforts. Currently physical removal of aboveground biomass is the primary means of controlling this weed, which is largely ineffective and does little to inhibit spread of the species. Little is known about reproduction of this species, although it is both rhizomatous and produces abundant seed. Here we report a series of studies looking at some of the basic reproductive mechanisms and strategies utilised by Saccharum spontaneum to provide information to support development of better targeted management strategies.
We found that seed produced between September and November was germinable both in the lab and in situ. Genetic diversity of mature stands was assessed using microsatellite markers and found to be high, even at small scales. Studies of vegetative reproduction showed that buds on stems that had been dried for up to six weeks were still capable of sprouting. Separate experiments showed that stem fragments could sprout when left on the surface or buried shallowly and that larger pieces sprouted more readily than smaller pieces.
Collectively these results demonstrate that Saccharum spontaneum in the PCW has the capability to produce many propagules that can successfully recruit and it is likely that seed dispersal drives the spread of the species. Timing of management actions to reduce flowering would significantly reduce the seed load into the environment and help to prevent spread to new sites. Similarly, where biomass is cut, cutting stems into smaller pieces will allow the stems to dry out and reduce the ability of buds to sprout. Additionally, attention should be paid to prevent accidental transport to new sites on machinery.
Invasive species, seed germination, asexual reproduction, microsatellite
Many of the “World’s Worst Weeds” are perennial species with the ability to spread with both seeds and vegetative structures (
Saccharum spontaneum L. (wild sugarcane; Poaceae) is a polymorphic species believed to have evolved in India (
In the Republic of Panamá, Saccharum spontaneum (Paja Canalera) has spread extensively in the Panama Canal Watershed (PCW) since the first herbarium specimen was collected in 1960 (MO1824369 J.E. Ebinger 490). It now dominates in abandoned agricultural lands and along human transportation corridors, such as roads and railroad tracks, encompassing over 3 percent of the watershed (
Flowering of Saccharum spontaneum across the landscape in Panama typically begins in August, midway through the rainy season which normally occurs from May through December. Inflorescences can be seen year-round, but are typically restricted to certain clones on a small scale during other times of the year (K. Saltonstall, pers. obs.). Flowering densities vary, averaging 4-5 stems/m2 during the peak flowering season (
The case for understanding the biology of invasive plants as a precursor to developing control strategies has been well made.
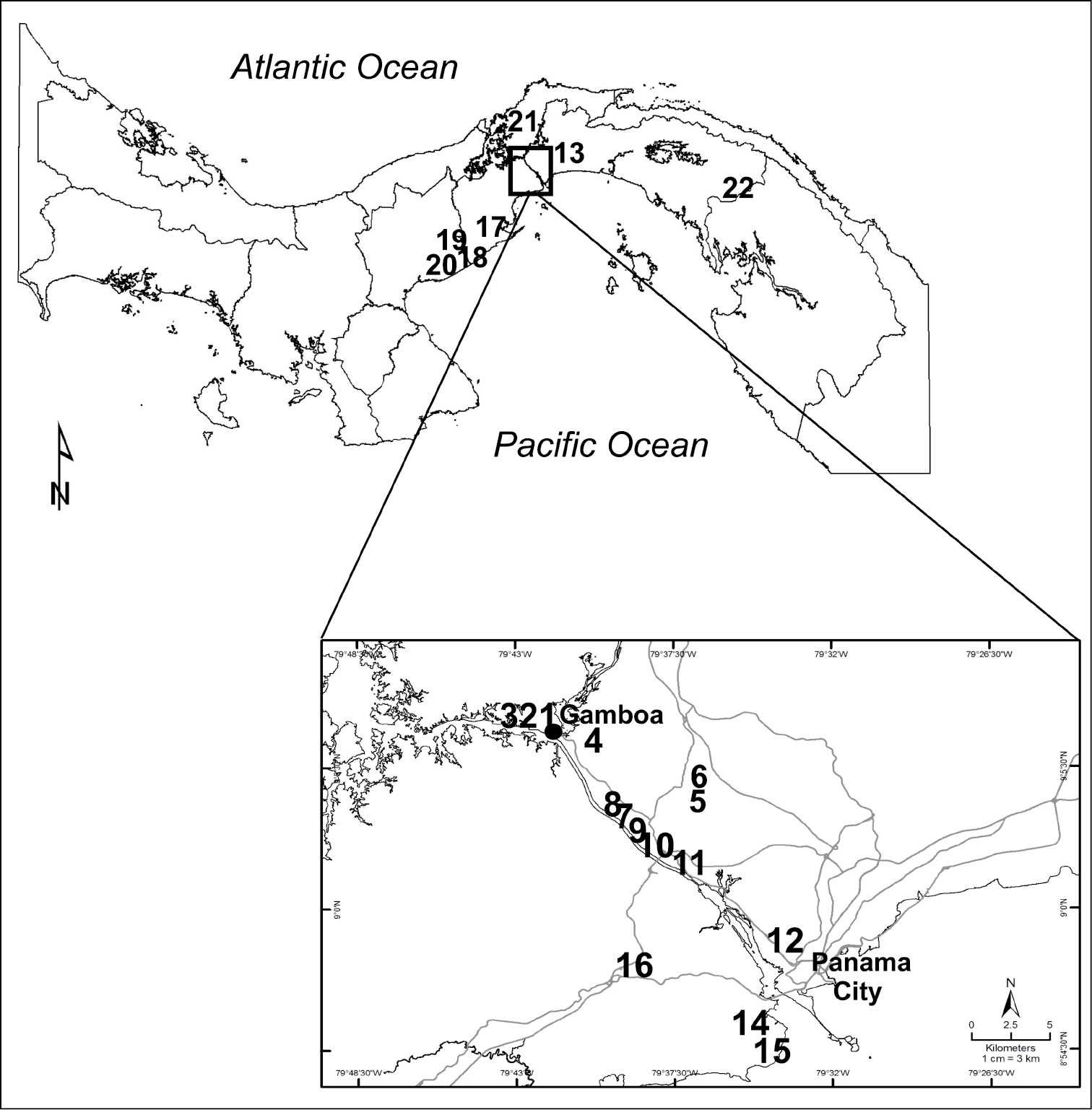
The Republic of Panamá is located at approximately 8–9° N latitude (Fig. 1), which is a latitude central to the native range of Saccharum spontaneum (
Map of Panama showing the locations of 22 sites where Saccharum spontaneum samples were collected. Seeds were collected weekly at Sites 1-12 for germination trials. Lines in the larger map indicate district boundaries. In the inset, the canal is shown between Panama City and Gamboa and major roads are indicated by grey lines.
We collected seeds of Saccharum spontaneum from 12 sites (Sites 1–12 Fig. 1; Appendix 1) in the PCW weekly between September and December. Each week three mature inflorescences, from different plants within a few meters of each other, were sampled and assayed separately. Seeds that had disarticulated from the inflorescence but remained loosely attached were collected, and kept at room temperature until assayed within two days of harvest. When wet at the time of harvest due to rain, seeds were dried under ambient conditions prior to conducting germination assays.
One hundred seeds were taken at random from those collected from each inflorescence and germinated on moist paper towel in petri dishes (n=3 inflorescences per site per week). Seeds were germinated at 36°C as Saccharum seeds have a very high temperature optimum for germination of around 36°C (
Germination was defined as root or radicle emergence visible to the naked eye. Seed germination was checked every three to four days and counted seedlings were removed from the plates. Total number of seeds germinated after two weeks is presented. For analysing the effect of date of collection on germination percentage we carried out a one-way ANOVA with date as a factor and log-transformed (log10(value+1)) germination data as the dependent variable (Sigmaplot 11, Systat Software Inc. San Jose, CA). Post –hoc comparisons of means were conducted using the Tukey method, for this and all subsequent analyses significance was tested at the 0.05 level.
To test if seeds would germinate in situ, vegetation was cleared and the soil surface raked on 16 September exposing areas of bare soil between two Saccharum spontaneum stands (Site 6, Fig. 1, Appendix 1). The experiment comprised 10 replicates of three treatments, each in 60 by 120 cm plots. Treatment one, bare soil, was designed to observe any seedlings emerging from seeds deposited by wind. The second treatment comprised six mature inflorescences placed on each plot and covered with nylon flywire to prevent seeds from blowing away. Plots in treatment three were bare soil covered by nylon flywire, with no additional seeds added. One replicate of each treatment was randomly placed in each of 10 evenly spaced blocks. After 19 days we removed the mesh to allow continued growth of seedlings. The number of germinated Saccharum spontaneum seedlings per plot was recorded one week after, and then every two weeks until nine weeks after the start of the experiment.
To test for differences in seedling numbers between treatments, we conducted a repeated measures analysis with time (within subject) and treatment (between subjects) as factors and the number of seedlings as the dependent variable (Sigmaplot 11). The number of seedlings was log-transformed (log10(value+1)) prior to analysis and post –hoc comparisons of means were conducted using the Holm-Sidak method (a variation of the Holm multi-comparison test using the Sidak correction (
To assess levels of genetic diversity within stands, plants were collected at 22 sites across central Panama, with a focus on the PCW but including the eastern- and westernmost edges of the invasion (Fig. 1, Appendix 1). Green leaves were harvested from individual culms spaced up to 10 m apart (n = 3 per site). To examine if the patterns we found at these sites were consistent across large stands, we also sampled parallel transects separated by 20 m at two independent stands in Soberania National Park within an area dominated by Saccharum spontaneum (Sites 5 and 6, Fig. 1, Appendix 1; see
We assessed multilocus allele profiles using 12 microsatellite loci (msscir14, msscir17, msscir53, msscir58, SMC28, SMC221, SMC334, SMC336, SMC597, SMC1047, SMC1237, SMC1493;
Effect of drying: To assess the ability of buds to remain viable and sprout after different periods of drying, culms were collected from Site 4 (Fig. 1, Appendix 1) on 3 September. Culms, approximately 2 m tall, that had not flowered were cut at the base and randomly assigned to five treatments (drying durations) spread out on benches that were shielded from rainfall but otherwise open. Drying durations were 0 days (day of harvest), one, two, four, and six weeks. After drying, stem pieces with a node at either end were cut from the culms and ten were placed into each of five replicates and covered with commercial peat. Each replicate comprised two plastic seedling trays (53 cm by 26 cm). Plants were grown under an open structure with a transparent roof, watered daily, and the plants that sprouted recorded four weeks after planting. To assess changes in moisture content, plant material was harvested from the same site on 19 November. Fresh and dry weights of stem pieces cut from culms at the time of harvest and after one, two and four weeks were recorded. Dry mass was taken after drying the stem pieces to constant weight at 40°C.
Data from the different times of drying were compared by ANOVA and times of drying were compared with Tukey multiple comparison tests (Sigmaplot 11). The proportion of buds that sprouted was square root transformed, other data was not transformed prior to analysis.
Fresh culms and rhizomes were collected at Site 6 (Fig. 1, Appendix 1) on July 3 and propagules were cut to the appropriate size and planted the same day. Both experiments were performed under a covered shelter, open on the sides. Unmodified soil representative of the area (Oxisol, collected in Rio Hato, Panama) was used and all pots were watered daily or as needed. All propagules were harvested on September 12 when the presence of a sprout above-ground, a sprout below-ground but not emerged, or the presence of roots was recorded.
Propagule size: Culm pieces with one node (and bud) were cut into either 2, 5, 10, or 20 cm lengths or two node (and bud) pieces ranging in length from 9.9 to 12.7 cm. Rhizomes were cut into pieces ranging from 15–40 mm in length, either with or without visible axillary buds. There were 10 replicates for each culm length (single and two node pieces) and 25 pieces of each rhizome type. Culm pieces shorter than 10 cm were planted individually in 10 cm diameter plastic nursery sacks while longer pieces were planted in 12 L pots. Rhizomes were planted individually in 10 cm sacks. All propagules were planted at a depth of 5 cm.
Planting depth: Individual propagules (5 cm single node stem pieces or single node rhizome pieces (ranging from 11–44 mm in length)) were planted at different depths in 10 cm nursery sacks. Planting depths were surface, 5 cm, 10 cm, and 20 cm for stem pieces, and surface, 10 cm, and 20 cm for rhizome pieces; there were ten replicates per planting depth. Time to sprouting was recorded for each propagule.
Our original intent was to analyse growth of sprouts using analysis of variance appropriate to the experimental design. However, because of the frequency of failure to sprout, and thus zero growth, we decided to analyse only the proportion sprouted either above- or belowground, as the percentage of the 10 replicates that sprouted for each treatment.
To test if soil type influenced the results of this experiment, growth was also tested using commercial potting mix in nursery sacks. Single node stem pieces (5 cm) were planted at the surface or 2 cm depth, in either commercial potting mix or unmodified soil from Rio Hato, with six replicates per treatment. Presence of sprouting was recorded after four weeks.
We further distinguish between propagules that produced roots at the node but did not initiate other growth, propagules that sprouted but died before they reached the soil surface, and propagules that produced visible growth above the soil surface. Results were analysed using generalized linear models (Poisson GLM for propagule depth and Binomial GLM for propagule size and soil type) as implemented in R 2.13.1 (R Development Core Team 2010). The proportion of variance explained by each model (r2) was calculated from the null and residual variances.
Germination ability
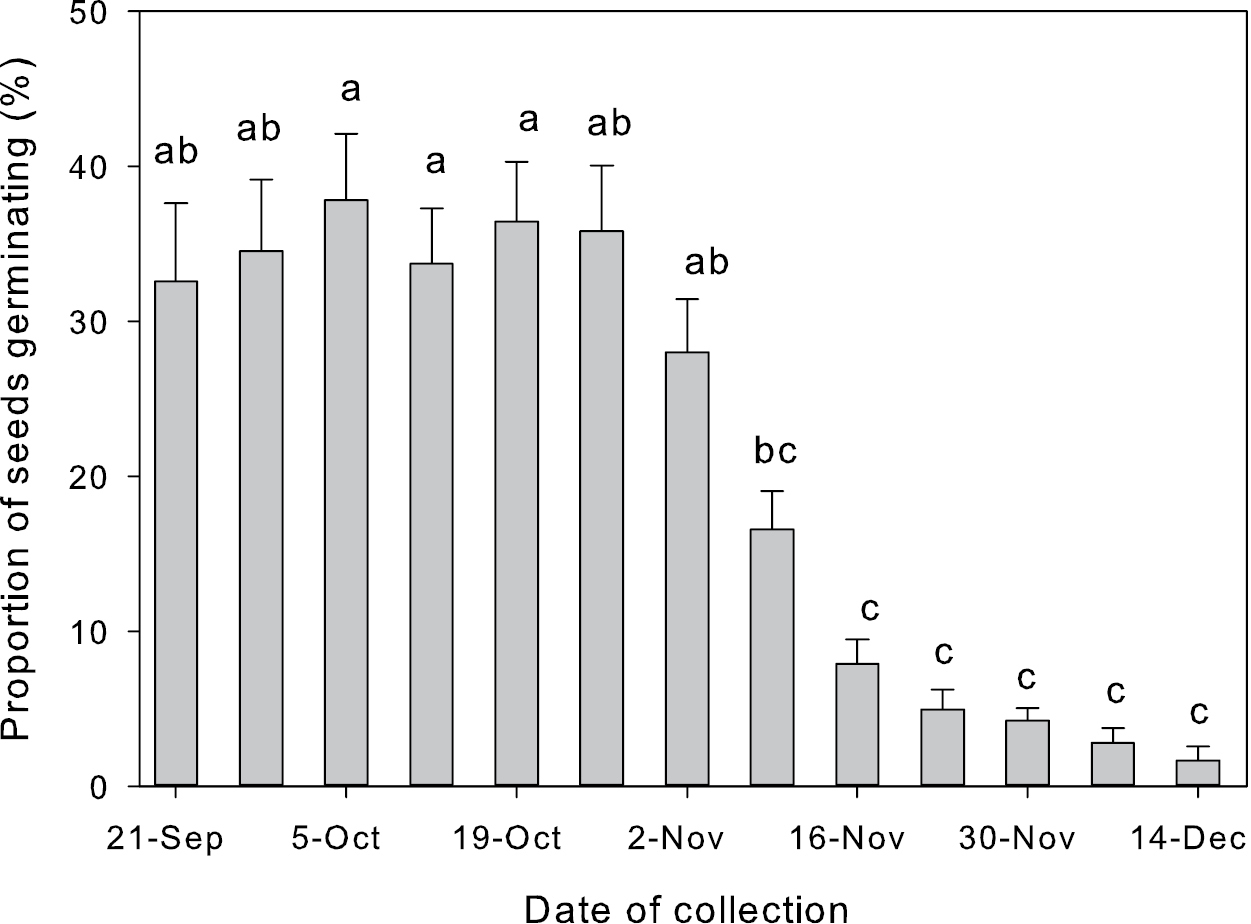
The proportion of seeds germinating and the temporal patterns of germination varied greatly across the 12 sites (Appendix 2). The range of maximum germination percentages were 72%–30% (Appendix 2), with an average level of germination around 35% across sites until November (Fig. 2). Significantly fewer seeds germinated after 16 November (ANOVA, F12, 403 = 25.5, P<0.001, Fig. 2).
Weekly average germination percentage of Saccharum spontaneum seeds from 12 sites. Error bars represent standard error of the mean. Dates of collection with different letters are significantly different.
In situ germination
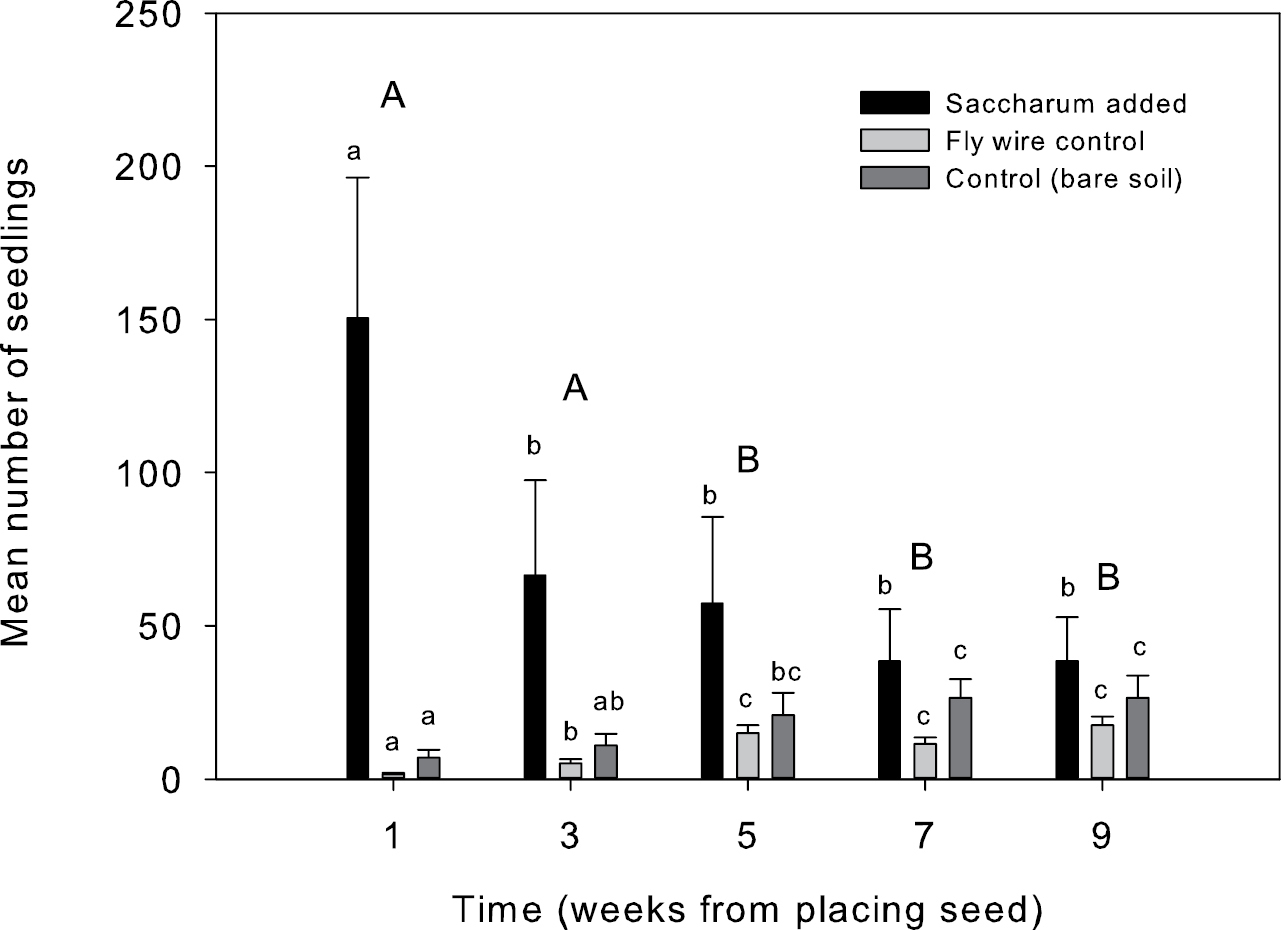
The numbers of seedlings germinating in situ showed a significant effect of treatment (F2, 18 = 21.6, p<0.001), week (F4, 36 = 7.4, p<0.001) and treatment by week interaction (F8, 72 = 29.9, p<0.001), so the effect of treatment was dependent upon time. One week after the experiment was initiated, germination was evident in all plots (Fig. 3) and there were significant differences in the number of seedlings between each treatment, with the plots with added seeds having many more seedlings than the controls. The average numbers of seedlings in plots with added seeds decreased from week to week, while those in control plots increased over time. Within the flywire treatment, there was a significant increase in the number of seedlings between weeks 3 and 5, corresponding with the removal of the flywire, whereas the seeds added and bare soil control plots did not show significant changes after week 3. After nine weeks there were no significant differences in the numbers of seedlings remaining between any of the treatments.
Average numbers of Saccharum spontaneum seedlings germinating each week per plot in the in situ germination experiment. “Saccharum added” plots had mature inflorescences placed in them and were initially covered with flywire, “flywire control” plots were initially covered with flywire but no seeds were added, and control plots were similar sized plots of bare soil. Error bars represent standard error of the mean (n=10). Weeks with different capital letters are significantly different from each other. Treatments with different small letters are significantly different from each other within that week.
Genetic diversity of mature stands
High levels of genetic diversity were found both within and across sites. A total of 227 alleles were observed across the twelve microsatellite loci, of which 215 were shared between sites. The average number of alleles per locus (Ao) was 18.4 and up to ten alleles per individual were found within a locus (range 1-10).
In the two stands sampled with transects, the majority of culms sampled were unique (Stand 1 at Site 5 = 89% and Stand 2 at Site 6 =65%) and all replicate allele phenotypes were found in adjacent plots. Similarly, in the 22 sites where only 3 samples were collected, 86% of them contained multiple allele phenotypes (19 of 22 sites). Fifty four multilocus allele profiles were found across the 22 sites, with three unique phenotypes sampled at each of 13 sites (Sites 3, 5, 6, 11, 13-16, and 18-22), two phenotypes at each of six sites (2, 4, 7, 9, 10, and 17), and a single phenotype at each of three sites (1, 8, and 12; Appendix 1). The same multilocus profile was not found at more than one site.
Effects of drying
There was a significant effect of time of drying of the culm on the proportion of buds that sprouted (ANOVA F4, 19 = 16.992, P<0.001, Table 1), less than half of the buds sprouting after 4 weeks. However while significant over time, the reduction in moisture content was only 8% lower after 4 weeks of drying (ANOVA F3, 16 = 7.819, P<0.01, Table 1).
Moisture content of stems and the proportion of buds that sprouted after various times of drying. Prior to testing for sprouting, buds were cut from culms immediately after harvesting (0) and 1–4 weeks of drying. Sprouting of buds was recorded after 4 weeks. Results are presented as the mean with the standard error in parenthesis. Values within a column with different letters are significantly different.
| Time (weeks) | Moisture content (%) | Proportion of buds sprouting (%) |
| 0 | 80.2 (1.08)a | 70.9 (4.39)ab |
| 1 | 76.7 (1.21)ab | 84.0 (2.92)a |
| 2 | 77.2 (0.68)ab | 57.0 (6.63)bc |
| 4 | 73.7 (0.74)b | 45.0 (3.16)c |
| 6 | N.D. | 38.8 (3.75)c |
N.D. = not determined
Effects of propagule size
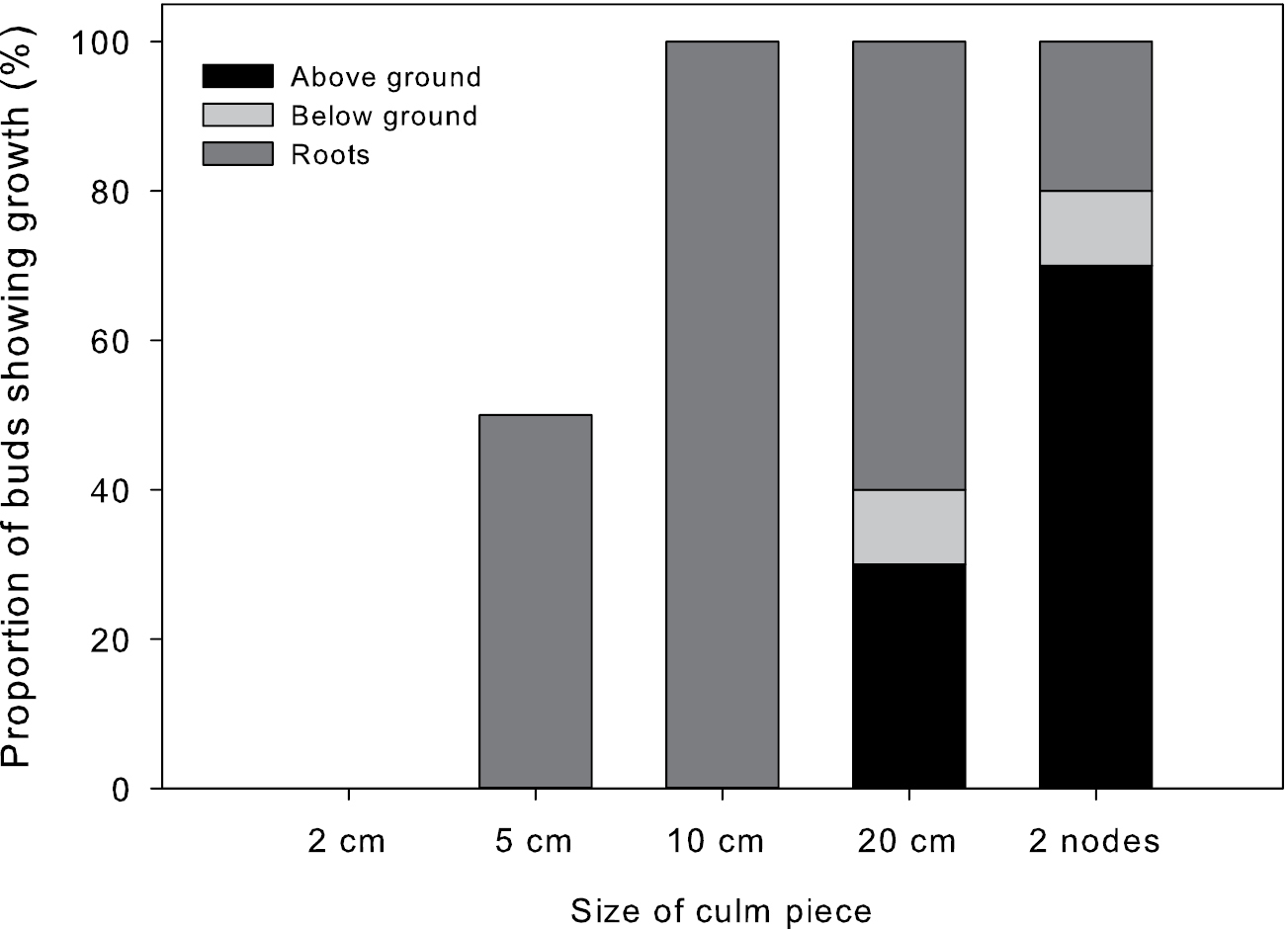
Increased size of stem fragments increased sprouting ability (p<0.01, r2 = 0.34; Fig. 5). While roots were produced in the majority of fragments of 5, 10 and 20 cm lengths, no fragments of 2 cm, 5 cm, or 10 cm produced active sprouts from the node. One 20 cm (10%) and one 2-node fragment (10%) sprouted belowground but died before reaching the soil surface. Three (30%) 20 cm fragments and seven (70%) 2-node fragments sprouted aboveground. All 2-node fragments that grew sprouted at only one node, but had roots present at both nodes. No rhizome fragments, with or without buds, sprouted.
Effects of planting depth
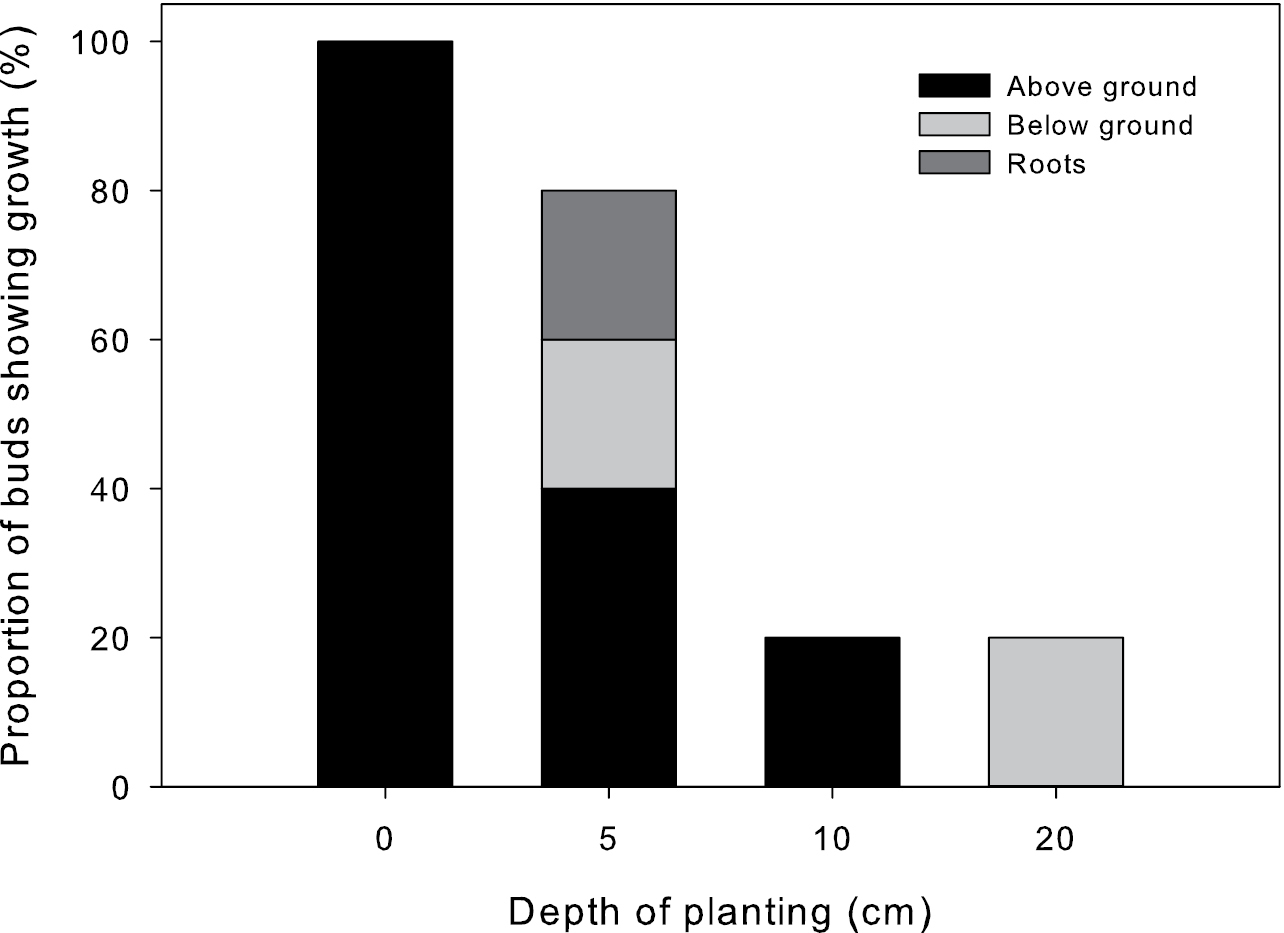
There was a significant negative effect of planting depth on the ability of stem cuttings to sprout, with lower depths having an inhibitory effect on sprouting (p<0.05, r2 = 0.29, Fig. 4). While 100% of stem cuttings laid at the soil surface sprouted, only 40% successfully grew aboveground from a depth of 5 cm and only 20% from 10 cm. Stems at a depth of 20 cm failed to grow aboveground, but 20% of propagules at this depth sprouted and died before reaching the soil surface. All rhizome pieces, at any depth, failed to sprout suggesting that single node pieces of Saccharum spontaneum rhizomes may have low sprouting ability (data not shown). One rhizome piece, at 10 cm depth, produced roots but did not produce a sprout. Rhizome pieces laid at the soil surface dried out quickly, despite daily watering, and did not even produce roots.
Depth trials comparing commercial potting soil (PS) to the unmodified soil (US) used in the previous trial showed similar results, with 83.3% (PS) and 67.7% (US) of cuttings laid at the soil surface sprouting aboveground and 67.5% (PS) and 50.0% (US) sprouting when planted at a depth of 2 cm, respectively. No significant differences were seen between depths or soil types (p>0.05, r2 = 0.05).
Proportion of buds sprouting from different depths of planting. Proportion of buds that produced visible growth above the soil surface (Above ground), that sprouted but died before they reached the soil surface (Below ground), or produced roots at the node (Roots), but did not initiate other growth as determined by depth of planting.
Proportion of buds sprouting from different sizes of culm piece planted. Proportion of buds that produced visible growth above the soil surface (above ground), that sprouted but died before they reached the soil surface (below ground), or produced roots at the node (roots), but did not initiate other growth as determined by size of culm piece.
The spread of Saccharum spontaneum in the PCW has been rapid and dramatic, occurring largely in recent decades. Flowering across the landscape is extensive and our results suggest that seed dispersal clearly plays a major role in this spread. Seed germination rates were high when tested under optimal conditions, and we found seedlings readily establish in situ. Although we did not follow the fate of these seedlings to maturity, 79% of our samples collected in mature stands had unique allele phenotypes, with all replicated genotypes occurring in adjacent samples within a site. Such high genotypic diversity cannot result from vegetative spread and can only be the result of sexual reproduction. Our trials with vegetative propagules also showed that growth can occur from stem fragments, although they can be highly sensitive to the conditions under which they are grown.
Although we did not test viability directly, our germination trials indicate that on a landscape scale large numbers of germinable seeds are produced annually. Variability in germination within and between sites was high but seeds germinated from all sites, particularly those collected during the peak flowering months of September and October. Other invasive grasses, such as Phragmites australis and Spartina alterniflora in North America, have also been shown to have highly variable production of viable seeds across sites (
Seeds which germinated in situ showed high mortality in plots where they were added, but control plots which received seeds only through natural dispersal increased their numbers of seedlings from week to week. In the case of the bare soil control plots, seedling densities were ~27 seedlings/plot (37 seedlings/m2) by the end of the experiment, which is similar to densities counted in plots measured in a Saccharum spontaneum stand which had burned earlier in the year (
Genetic diversity across the landscape is high. We found high numbers of alleles both within individuals and between sites, and nearly all samples tested were genetically unique. This suggests that stands of Saccharum spontaneum typically form from the coalescence of multiple individuals recruited from seeds rather than vegetative propagation of a single clone across a large area. Consequently, though we have not demonstrated recruitment of adult plants from seedlings, flowering is a life-history stage that needs to be targeted to effectively manage the spread of the species.
The effects of disconnection of stems and rhizomes on vegetative reproduction has been studied extensively in rhizomatous grasses, and disconnection from the parent plant has been shown to release buds from inhibition by other buds on the stem or rhizome. This response may be influenced by the length of the segment containing the buds as well as which the conditions to which propagules may be exposed to following disconnection from the parent plant (
Ability of buds to sprout also appears to be strongly affected by planting depth. Planting stem cuttings at depths as shallow as 5 cm reduced the number of aboveground sprouts by 60%, and deeper plantings inhibited even the sprouting of fine roots from the nodes. The lack of growth in our initial studies prompted concerns about the soil that we used inhibiting growth in this experiment. Although the bags we used had drainage holes, the Oxisols used here have high clay content and waterlogging may have been an issue preventing growth of propagules. However, our secondary trials comparing growth in the Oxisol versus commercial potting mix showed similar results, with reduced growth when fragments were planted below the soil surface suggesting that the inhibition of growth could be a light response rather than an effect of the growth substrate.
Management of Saccharum spontaneum in Panama is restricted to controlling aboveground biomass, and generally involves physical cutting of mature biomass, and in some cases, chemical control where reforestation is a goal (
Timing and method of control thus become important when considering management of this aggressive invader in Panama. While intermittent flowering of Saccharum spontaneum occurs year round, the peak season of flowering is from August – October. Our studies have shown that seed germinability is highest in the months of September, October, and early November with a rapid decline in germinability in December. We suspect that germinability of seeds produced at other times of the year is also much lower. Timing of biomass removal can be important, with removal in June or July, prior to inflorescence emergence and seed development but late enough in the year that reproduction will not occur in the months of August – November, possibly being a method to reduce spread by seed. This may be particularly important in areas on the edges of the distribution where Saccharum spontaneum does not yet dominate and spread can be minimized on a local scale. As herbicides are not commonly used in Panama except during the initial stages of reforestation projects, repeated cutting of stems throughout the year to deplete below-ground carbohydrate resources in rhizomes is another approach which may help to prevent flowering at optimal times of the year. However, as our studies have shown that cut stems are still capable of sprouting after six weeks of desiccation, cut biomass should also be handled to minimize sprouting and spread to new sites.
Better control of dry-season fires is also needed to help control spread of Saccharum spontaneum by seed, as rapid regrowth following the onset of the rainy season typically leads to stems with mature infloresences developing in the peak months of seed production. Further, burned areas have higher densities of flowering stems and comparable seed germination rates as unburned areas (
As Saccharum spontaneum in Central Panama is widespread and many thousands of seeds are produced by each plant, a strategy to control seed numbers will have to be co-ordinated over a large area to be truly effective. While the extent of seed dispersal has not been experimentally quantified, research conducted on Barro Colorado Island (BCI) in the Panama Canal, utilising 200 seed rain traps (
In conclusion we have presented evidence that the spread of Saccharum spontaneum in Panama has been driven by seed production, but that vegetative propagules derived from stem fragments are also robust and can be the source of new plants. Seed production from Saccharum spontaneum can be reduced through the timing of management actions to reduce biomass that prevents re-growth of flowering stems. However the mobility of the seed means that to be effective, such actions would have to be conducted over large areas. Once established, the persistence of individual stands through re-growth from buds lower on the stems and rhizomes will continue to impede control and eradication over large areas so further research to determine a weak point in the vegetative growth stage is needed.
GDB thanks the Queensland Government (Queensland Smithsonian Fellowship), CRC Sugar Industry Innovation through Biotechnology and the Australian Government and the Australian Sugarcane Industry as provided by the Sugar Research and Development Corporation for financial support for an extended visit to the Smithsonian Tropical Research Institute. KS was funded by a Smithsonian Postdoctoral Fellowship. We thank the Smithsonian Tropical Research Institute and Jefferson Hall for logistical support, Eldredge Bermingham for the use of his laboratory, and Milton Salano for assistance with Fig. 1. Deanne Haffner is thanked for extensive input to the germination assays. J. Hall and K. Juneau provided helpful comments on earlier drafts of the manuscript.
Locations, description and diversity of the sites used for the collection of Saccharum spontaneum seeds. (doi: 10.3897/neobiota.20.6163.app1) File format: Adobe PDF file (pdf).
Explanation note: The table gives the latitude and longitude, description and number of genotypes found among the 3 plants of Saccharum spontaneum tested from each of 22 Sites. Sites 1–12 were used to assess seed germinability through time.
Germinability of Saccharum spontaneum seed through time from 12 sites. (doi: 10.3897/neobiota.20.6163.app2) File format: Adobe PDF file (pdf).
Explanation note: Proportion of seeds that germinated each week between September and December from samples taken at 12 sites. 100 seeds were germinated from each of three replicate samples and tested for germination in laboratory conditions. Results are presented as the mean and the error bar represents the standard error of the mean.