Citation: Abbas AM, Rubio-Casal AE, de Cires A, Figueroa E, Nieva JJ, Castillo JM (2014) Wrack burial reduces germination and establishment of the invasive cordgrass Spartina densiflora. In: Capdevila-Argüelles L, Zilletti B (Eds) Proceedings of 7th NEOBIOTA conference, Pontevedra, Spain. NeoBiota 21: 65–79. doi: 10.3897/neobiota.21.4963
Germination and emergence of halophytes may decrease significantly by seed burial in dead plant material, or wrack, which is common and abundant in tidal marshes. The effects of plant debris (wrack) burial on seed germination and seedling establishment of Spartina densiflora, an invasive cordgrass, were studied under greenhouse conditions and compared with field observations. Five wrack burial depths were applied: control without wrack, 1 cm (1235 ± 92 g DW wrack m-2), 2 cm (3266 ± 13 g DW m-2), 4 cm (4213 ± 277 g DW m-2), and 8 cm (6138 ± 227 g DW m-2). Sediment pH, electrical conductivity, redox potential and temperature were recorded. Quiescence increased with wrack load up to ~20% at 8 cm deep. Germination decreased with wrack load from 96% to 14%, which could be related with anoxic conditions under the debris since sediment redox potential was as low as -83 ± 7 mV at 8 cm. Germination percentage increased and quiescent and dormant percentages decreased at higher daily sediment temperatures and with higher daily temperature fluctuations, conditions that were recorded without or under low loads of wrack. Spartina densiflora did not show primary dormancy, but its seeds entered into a non-deep physiological dormancy below 1 cm deep in plant debris. The establishment of S. densiflora seedlings was also greatly reduced by wrack burial since only 6 seedlings (11 ± 5 % of germinated seeds) emerged above plant debris from 1 cm and all seedlings died from deeper than 1 cm. S. densiflora seedling development was also reduced by wrack burial. The inverse relationship between germination and emergence of S. densiflora with wrack burial recorded in our study is useful to predict its invasion dynamics and to plan the management of invaded marshes.
Coastal marshes, physiological dormancy, plant debris, seedling, Spartina densiflora
Most salt marsh environments are unpredictable for plants, because of the occurrence of both salinity and flooding stress (
Rafts of dead plant material, or wrack, are common and abundant in tidal marshes. Due to high primary productivity rates and low consumption rates by herbivorous, halophytes, such as eelgrasses and cordgrasses, produce high quantities of wrack, especially in dye-back areas. This wrack is added up to that transported by rivers to salt marshes in estuaries. Then, tidal creeks provide corridors for wrack transport into salt marshes where it can disturb plant communities (
The austral cordgrass, Spartina densiflora Brongn. (Poaceae) is invading salt marshes in southern Europe, Northwest Africa and the West Coast of North America (
While cordgrasses (Spartina genus) are one of the most abundant and frequent halophytes in salt marshes, there have not been any studies that examined how their seeds and seedlings respond to wrack burial. We examined under controlled greenhouse conditions the impact of five wrack burial depths (0, 1, 2, 4 and 8 cm) on germination, seed viability and seedling establishment of Spartina densiflora. Main abiotic factors conditioning seed germination and seedling establishment in salt marshes (sediment pH, electrical conductivity, redox potential and temperature) were also recorded for every wrack treatment. We hypothesized that Spartina densiflora germination and establishment would be reduced by wrack burial due to anoxia and/or low temperature fluctuations.
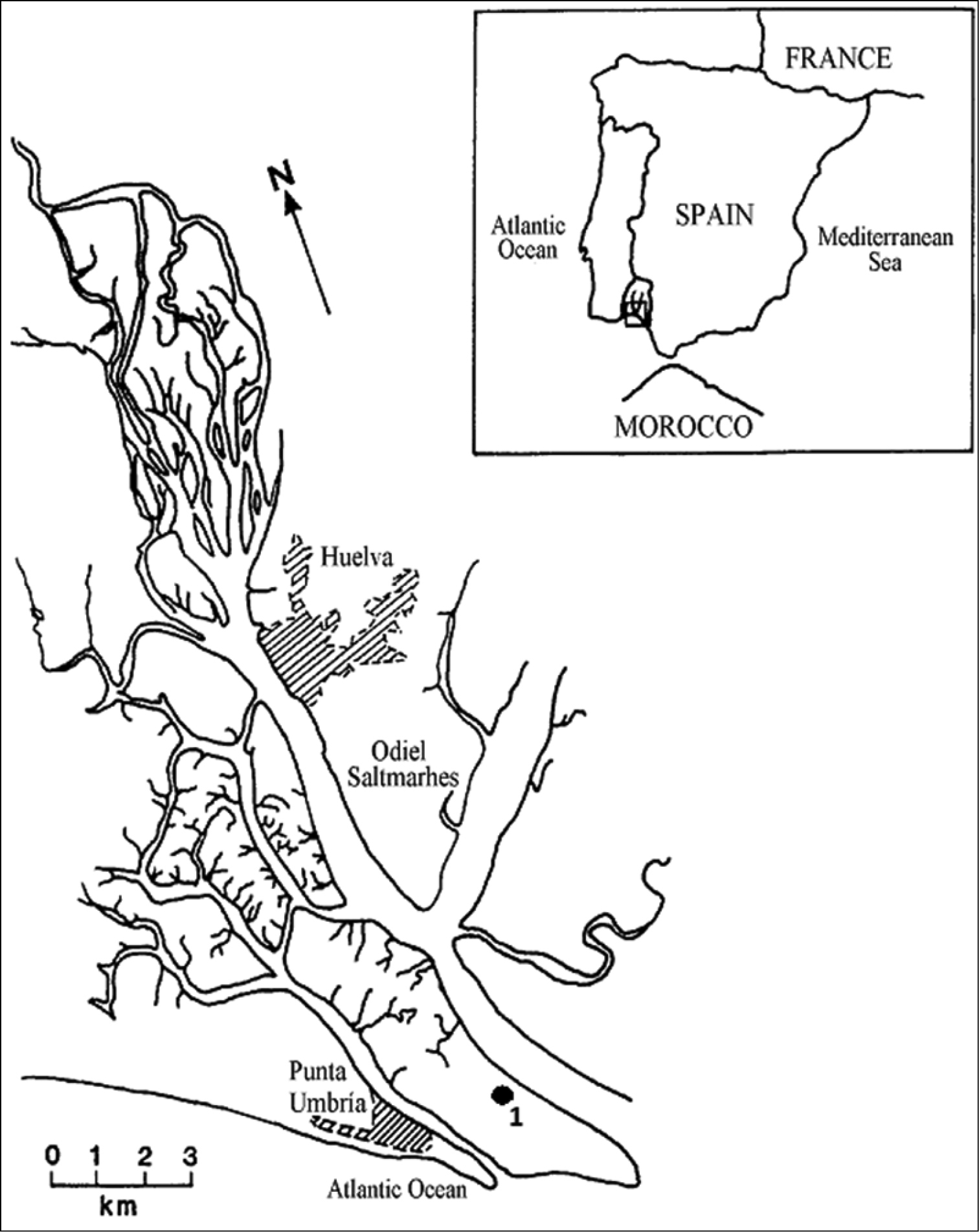
Spartina densiflora seeds were collected in August 2009 from multiple mature individuals chosen at random from a population growing at the periphery of an accreting, well-drained intertidal lagoon at Odiel Marshes (Southwest Iberian Peninsula; 37°08' - 37°20'N, 6°45' - 7°02'W; Fig. 1); see
Location of the Odiel Marshes on the Atlantic coast of Southwest Iberian Peninsula (37°08'-37°20'N, 6°45'-7°02'W), and the sampling point where Spartina densiflora seeds were collected (1).
A greenhouse experiment was conducted from December 2009 to February 2010, since most Spartina densiflora seeds germinate during winter, to test the effects of wrack burial on seed germination, and emergence and growth of seedlings of Spartina densiflora. The mean disseminule size of Spartina densiflora was 9.67 ± 0.15 mm by 1.47 ± 0.02 mm (n = 50) and its mean weight was 3.0 ± 0.1 mg (range: 1.3-4.2 mg). Mean Spartina caryopsis size was 4.68 ± 0.08 mm by 0.98 ± 0.02 mm (n = 50) and its weight was 2.0 ± 0.1 mg (range: 1.3–3.0 mg). Four replicates of 25 seeds were sown at 1 cm depth in clean sand in plastic containers measuring 18 cm width, 22 cm length and 11 cm height (containing ~1.6 kg of clean sand). The sand was collected from the same marsh where the seeds were obtained and it was sieved through 0.5-mm mesh size filling to eliminate pre-existing seeds and other plant material. A control treatment was set up without any seeds added in order to test whether the sand we used contained seeds. Five wrack burial treatments were conducted: control (no wrack was added above the sand surface), 1 cm (1235 ± 92 g DW wrack m-2), 2 cm (3266 ± 13 g DW m-2), 4 cm (4213 ± 277 g DW m-2), and 8 cm (6138 ± 227 g DW m-2) of wrack burial depth. These treatments were decided following our field observations in Odiel Marshes (Southwest Iberian Peninsula) where Spartina densiflora is very abundant (
Sediment redox potential, electrical conductivity and pH were recorded at the end of the experiment in February 2010 at 1 cm depth in the sand. pH was recorded in the laboratory after adding distilled water to the soil (1:1, soil: distilled water, v/v) (pH/redox Crison with the electrode M-506). Soil salinity was measured as electrical conductivity (conductivity meter, Crison-522) after pH (1:2, soil: distilled water, v/v)). Redox potential was determined with a portable meter and electrode system in the greenhouse (Crison pH/mV p-506). Mean, maximum and minimum daily sediment temperature were recorded at 0, 1, 2, 4 and 8 cm depths using an electronic thermometer (SA880SSX, Germany) recording from 8 a.m. to 8 p.m. every 4 hours for 3 days (n = 3). Average daily air temperature during the experiment was 18.5 ± 0.5 °C, varying between a mean low temperatures of 10.4 ± 1.6 °C and a mean maximum temperature of 34 ± 3.5 °C. Mean daily air relative humidity was 74.5 ± 2.6%, varying between 32.5 ± 5.5% and 92.5 ± 1.1%.
Emerged seedlings from beneath the wrack were counted every 24 h (
Ungerminated seeds were transferred to 0.5-cm depth in sand and germination and emergence percentages were recorded until no more seeds germinated for 10 days. Germinated seeds were considered to be in a quiescent state, which is different than true seed dormancy and occurs when a seed fails to germinate because external environmental conditions are not appropriate (
Analyses were carried out using SPSS 12.0 (SPSS Inc, USA). Data were tested for homogeneity of variance and normality with the Brown-Forsythe test and the Kolmogorov-Smirnov test, respectively (P < 0.05). Plant traits were compared between treatments by one-way analysis of variance (ANOVA, F-test). Tukey Honest Significant Difference (HSD) test between means was calculated only if the F-test was significant (P < 0.05). The effect of wrack load on seedling growth rate between two treatments was analyzed using a Student t- test. Pearson correlation coefficient and linear regressions were calculated between abiotic factors, germination and seedling traits, and the wrack load. When a biotic characteristic was correlated with two or more abiotic environmental factors, multiple regression analysis was carried out to explore relative weights (β). Deviations were calculated as standard errors of the mean (SEM).
Sediment pH increased with the wrack burial load from 6.2 ± 0.5 in the control treatment to 7.7 ± 0.1 under 8 cm (r = 0.96, P < 0.01, n = 20). Electrical conductivity changed from 0.11 ± 0.00 to 0.23 ± 0.00 mS cm-1. Sediment redox potential varied between -83 ± 7 mV under 8 cm of wrack and +255 ± 5 mV without wrack burial (Table 1). Mean daily sediment temperature decreased at higher depths (r = -0.61, P < 0.05, n = 20), but without showing significant differences between treatments, varying between 12.3 ± 1.0 °C for the control treatment and 10.1 ± 0.8 °C at 4 and 8 cm (ANOVA, F = 0.39, P > 0.05). Maximum daily sediment temperature changed between +25.0 ± 0.8 °C for the control treatment and +21.7 ± 0.3 °C at 1 cm (ANOVA, F = 2.02, P > 0.05), decreasing also when wrack burial depth increased (r = -0.36, P > 0.05, n = 20). Minimum daily temperature varied between +10.4 ± 0.4 °C at 8 cm and +7.9 ± 1.0 °C for the control treatment (ANOVA, F = 0.86, P > 0.05), increasing with depth (r = 0.81, P < 0.0001, n = 20). Daily variation between maximum and minimum temperatures decreased at higher depths (r = -0.62, P < 0.05, n = 20), varying between 17.7 ± 1.4 °C for the control treatment and 12.4 ± 0.3 °C at 8 cm (ANOVA, F = 2.46, P < 0.05) (Table 1).
Wrack load (g m-2), sediment pH, redox potential (mV), electrical conductivity (mS cm-1), daily mean, maximum and minimum sediment temperature (°C), and the difference between maximum and minimum temperature (°) (n = 3) for five wrack burial depths. Different letters indicate significant differences between treatments (ANOVA, P < 0.05) (n = 5).
| Wrack depth (cm) | Wrack load (g m-2) | pH | Redox potential (mV) | Conductivity (mS cm-1) | Daily sediment temperature (°C) | |||
|---|---|---|---|---|---|---|---|---|
| Mean | Maximum | Minimum | Max-Min | |||||
| 0 | 0 ± 0a | 6.2 ± 0.5a | +255 ± 5a | 0.23 ± 0.00a | 12.3 ± 1.0a | 25.0 ± 0.8a | 7.9 ± 1.0a | 17.7 ± 0.2a |
| 1 | 1235 ± 92b | 6.9 ± 0.3b | +247 ± 2a | 0.20 ± 0.02a | 10.4 ± 0.9a | 21.7 ± 0.3a | 9.4 ± 0.5a | 13.0 ± 0.3b |
| 2 | 3266 ± 13c | 7.1 ± 0.5b | +229 ± 3b | 0.16 ± 0.00b | 10.2 ± 0.9a | 22.5 ± 0.1a | 9.7 ± 0.5a | 13.1 ± 0.0b |
| 4 | 4213 ± 277d | 7.3 ± 0.1c | +157 ± 11c | 0.13 ± 0.01c | 10.1 ± 0.8a | 22.7 ± 0.8a | 10.1 ± 0.4a | 12.7 ± 0.1b |
| 8 | 6138 ± 227e | 7.7 ± 0.1d | -83 ± 7d | 0.11 ± 0.00d | 10.1 ± 0.8a | 22.5 ± 0.0a | 10.4 ± 0.4a | 12.4 ± 0.3b |
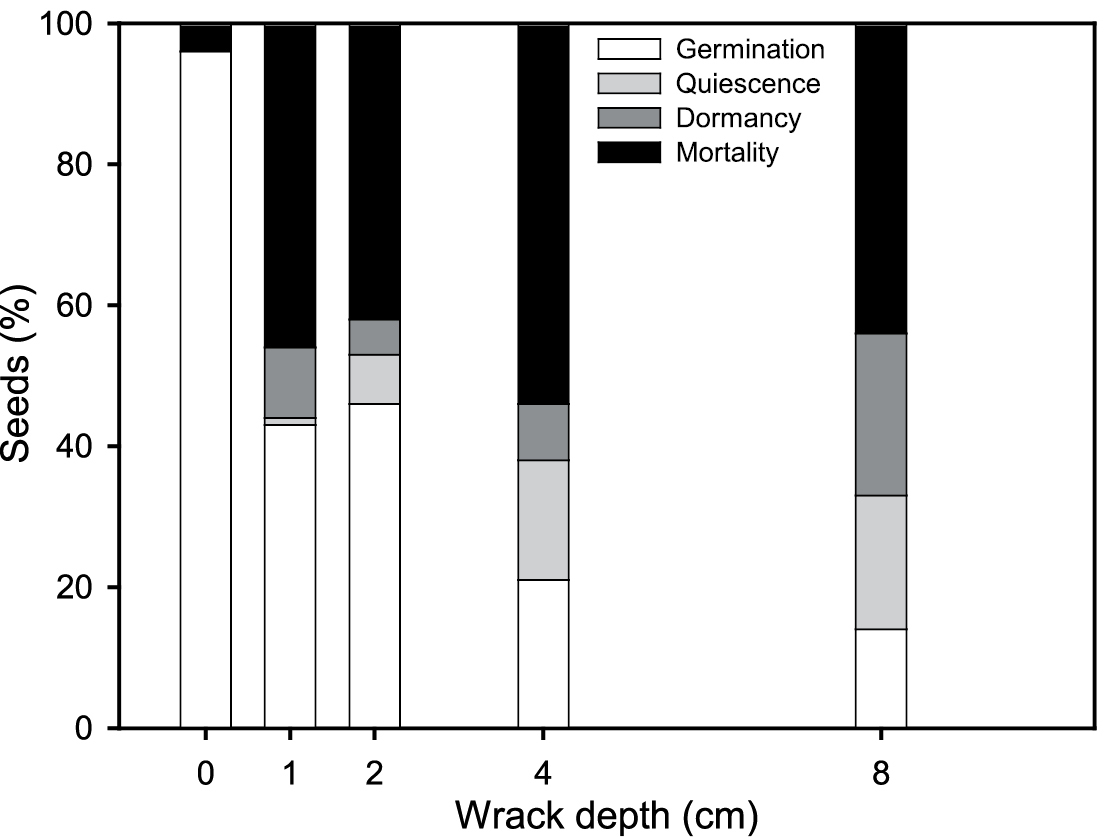
The time to first emergence of Spartina densiflora was 23 days after the start of the experiment at control treatment and 37 days at 1 cm wrack depth. Germination decreased when wrack load increased (r = -0.84, P < 0.0001, n = 20), showing the highest value without wrack (96 ± 4%) and decreasing significantly under 8 cm deep (ANOVA, P < 0.05) (Fig. 2).
Germination, quiescence, dormancy and mortality of Spartina densiflora seeds for five wrack burial depths.
Germination percentage decreased at lower redox potentials (r = 0.65, P < 0.005, n = 20; β = -0.599), varying from 96 ± 4% at the control treatment with sediment redox potential +255 ± 5 mV to less than 15% under 8 cm deep with negative redox potential -83 ± 7 mV. In addition, germination percentage increased at lower minimum daily sediment temperature (r = -0.95, P < 0.0001, n = 20; β = -3.669) (Fig. 2).
Quiescence and dormancy percentages increased with wrack load (r = 0.67, P< 0.05, n = 20; r = 0.70, P < 0.001, n = 20, respectively). Quiescent percentage increased at higher minimum daily sediment temperature (r = 0.57, P < 0.05, n = 20; β = 3.265) and at lower redox potentials (r = -0.56, P < 0.01, n = 20; β = 0.495). No dormant seeds were recorded for the control treatment where seed mortality was the lowest (4%; 4 seeds). Dormancy and mortality increased to ~50% of the ungerminated seeds under wrack (Fig. 2). Dormant seed percentage and seed mortality increased mainly at higher daily minimum sediment temperature (r = 0.70, P < 0.001, n = 20; r = 0.81, P < 0.0001, n = 20, respectively).
No seedling emerged from deeper than 1 cm depth since every seedling died before emerging above the wrack surface. Minimum seedling mortality was recorded for the control treatment (7 ± 3% of germinated seeds) (ANOVA, P < 0.05). The highest seedling emergence percentage occurred without wrack (93 ± 3%) and only 6 seedlings emerged from under 1 cm of wrack (11 ± 5%) (Table 2).
Emergence and mortality rates of germinated seeds for five wrack burial depths. Different letters indicate significant differences between wrack burial depths for the same trait (ANOVA, P < 0.05).
| Wrack depth (cm) | Emergence (%) | Mortality (%) |
|---|---|---|
| 0 | 93 ± 3a | 7 ± 3a |
| 1 | 11 ± 5b | 89 ± 5b |
| 2 | 0 ± 0c | 100 ± 0c |
| 4 | 0 ± 0c | 100 ± 0c |
| 8 | 0 ± 0c | 100 ± 0c |
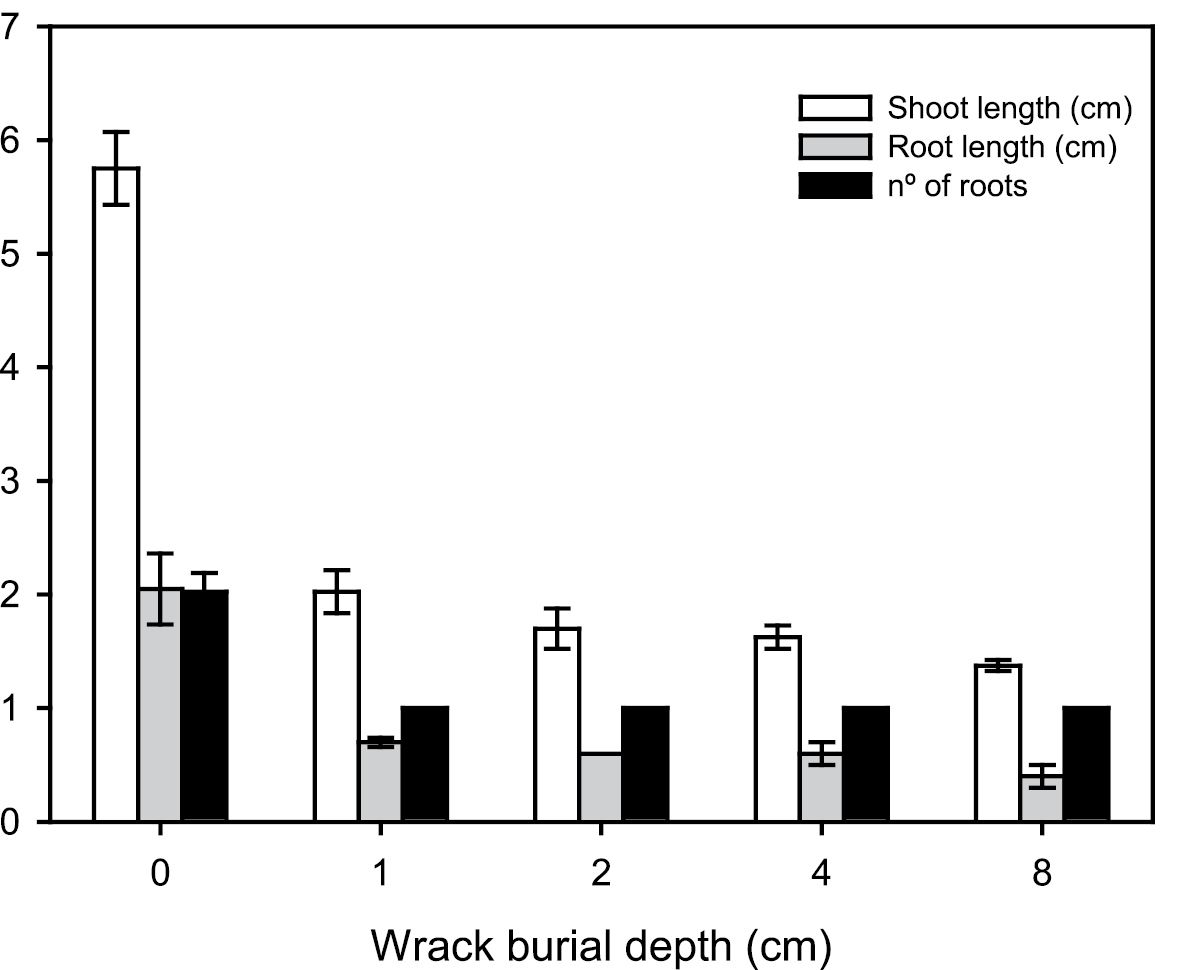
Seedlings in the control treatment were much taller and had longer and more roots than those growing from under 1 cm of wrack and also than those seedlings dying under the wrack at 2, 4 and 8 cm (Fig. 3). These results also showed a higher aerial growth rate for seedlings at the control treatment (0.11 ± 0.01 cm day-1) than those growing from under 1 cm of wrack (0.02 ± 0.01 cm day-1) (t-test; P < 0.05).
Shoot and root lengths (cm) and root number of Spartina densiflora seedlings for five wrack burial depths (seedlings from deeper than 1 cm were found dead and did not emerge above the wrack surface). Different letters indicate significant differences between wrack depths for the same trait (ANOVA, P < 0.05).
This study shows that germination and establishment of Spartina densiflora, an invasive cordgrass in Europe, North America and North Africa, is greatly limited by wrack burial.
Sediment pH increased with wrack burial load, which may be related to the presence of shells in the debris that would add carbonate to the sediment. However, Spartina densiflora germination would not be altered within the narrow recorded pH range (6.2–7.7) (
The highest germination was recorded without debris (96%), decreasing markedly (to values between 21–43%) between 1 and 4 cm deep with positive redox potentials (> +150 mV). Therefore, other environmental factors in addition to anoxia seemed to be limiting Spartina densiflora germination under the debris. The effects of burial on germination can be mediated by changes in the light regime as it has been described for some halophytes (
Spartina densiflora did not show primary dormancy since all its seeds germinated or died (only 4%) without wrack in optimal conditions. Instead, Spartina densiflora seeds entered a non-deep physiological dormancy (
The establishment of Spartina densiflora seedlings was also greatly influenced by wrack. Only six seedlings emerged above the plant debris from a burial depth of 1 cm, while no seedling emerged from deeper than 1 cm (wrack load > 3 kg DW m-2). The seedling of Spartina densiflora has just one thin and sharp cotyledon that grows easily along an axis, being able to emerge straight away from 4 cm depth in water (
Spartina densiflora seedling development was also significantly reduced by wrack burial. Seedlings growing without wrack were ~5.8 cm tall at the end of the experiment while seedlings emerging from 1 cm deep under the wrack were ~2 cm tall (including their buried part), coinciding with lower growth rates. In adittion, seedlings in the control treatment had longer and more roots. In this sense, it has been reported that phytotoxins generated in anaerobic decomposition can inhibit the growth of freshwater plants (
Our experimental results are in accordance with our field observations. In tidal salt marshes, wrack is accumulated mainly coinciding with the mean higher high water. We have observed in the field (Odiel Marshes) that the wrack depth in these areas ranges from 2 to 14 cm. In these marshes, we have seen no Spartina densiflora seedling growing from within the wrack. Just very few Spartina adult clumps were observed within the debris areas, which seemed to have established before wrack accumulation or within open patches in the wrack. Spartina densiflora tussocks accumulate high densities of dead tillers in middle and high marshes (
Data gathered during this study confirmed an inverse relationship between germination and emergence with wrack burial for the invasive cordgrass Spartina densiflora. Germination decreased from 96% without wrack to 14% at 8 cm deep in debris (ca. 6 kg DW m-2). No seedling emerged above the wrack surface for seeds germinated at wrack burial depths greater than 1 cm (a wrack load of ca. 1 kg DW m-2). The results from this study improved our understanding of Spartina densiflora invasion and they are useful to predict invasion dynamics and to plan the management of invaded marshes. Thus, wrack may be used to limit Spartina densiflora colonization and should not be removed from those areas sensible to the invasion of this cordgrass.
This work was supported by a Ph.D scholarship of Egyptian Government-Ministry of Higher Education (cultural affairs and missions sector). We thank Jesús and Jose at the Greenhouse Facility of the University of Seville.