Citation: Kaiser-Bunbury CN, Cuthbert H, Fox R, Darryl Birch, Bunbury N (2014) Invasion of yellow crazy ant Anoplolepis gracilipes in a Seychelles UNESCO palm forest. NeoBiota 22: 43–57. doi: 10.3897/neobiota.22.6634
The mature palm forest of the Vallée de Mai, a UNESCO World Heritage Site, on the Seychelles island of Praslin, is a unique ecosystem containing many endemic species, including the iconic coco de mer palm Lodoicea maldivica. In 2009, the invasive yellow crazy ant Anoplolepis gracilipes was recorded for the first time within the palm forest, raising concern about its potential impacts on the endemic fauna. This research aimed to: (1) assess the current distribution and spread of A. gracilipes within the palm forest; (2) identify environmental variables that are linked to A. gracilipes distribution; and (3) compare endemic species richness and abundance in A. gracilipes invaded and uninvaded areas. Anoplolepis gracilipes was confined to the north-east of the site and remained almost stationary between April 2010 and December 2012, with isolated outbreaks into the forest. Infested areas had significantly higher temperature and humidity and lower canopy cover. Abundance and species richness of the endemic arboreal fauna were lower in the A. gracilipes invaded area. Molluscs were absent from the invaded area. The current restricted distribution of A. gracilipes in this ecosystem, combined with lower abundance of endemic fauna in the invaded area, highlight the need for further research to assess control measures and the possible role of biotic resistance to the invasion of the palm forest by A. gracilipes.
Endemic arboreal species, coco de mer palm, geckos, invasive alien species, islands, molluscs, World Heritage Site, Western Indian Ocean
Ants are highly successful invaders, particularly on islands (
In the Seychelles, Anoplolepis gracilipes was first recorded in 1962 on the main island of Mahé (
Although the impacts and ecology of Anoplolepis gracilipes have been well documented in degraded habitats in the Seychelles (
Anoplolepis gracilipes was identified in the Vallée de Mai for the first time in August 2009 (L. Chong-Seng & P. Matyot, pers. comm.). Here, we present research into the distribution of Anoplolepis gracilipes over the subsequent 2.5 year period in the Vallée de Mai palm forest, and its potential impact on a key group of animals in the ecosystem, the arboreal vertebrate and invertebrate palm specialist species. The overall aim of this research was to determine the distribution and spread of Anoplolepis gracilipes in the Vallée de Mai over time and improve understanding of its impact on endemic arboreal species in this unique palm forest. Given the extent of Anoplolepis gracilipes’ impacts elsewhere, we expected that the fauna associated with Lodoicea maldivica would be less abundant where Anoplolepis gracilipes was present. We specifically ask: (1) what are the distribution, spread rate, and activity levels of Anoplolepis gracilipes in the palm forest?; (2) Which environmental variables are associated with Anoplolepis gracilipes distribution?; and (3) Are there differences in the number of species and abundance of endemic arboreal fauna between Anoplolepis gracilipes invaded and uninvaded areas of the Vallée de Mai?
The study was conducted in the Vallée de Mai (19.5 ha; 4°19'S, 55°44'E) which is located in Praslin National Park (342 ha; Fig. 1). The Vallée de Mai was inscribed as a natural UNESCO World Heritage Site in 1983 for its unique and globally important habitat. The vegetation consists of low-intermediate elevation palm forest dominated by Lodoicea maldivica. A strip of cleared vegetation with trimmed introduced and native trees is maintained around the area as a firebreak. Outside the firebreak, the vegetation is mixed with more native and introduced broadleaf species. There is a network of paths used by visitors throughout the Vallée de Mai which are regularly swept and kept free of leaf litter. The Seychelles has a tropical climate and experiences temperatures of 24–32 °C and average rainfall of ca. 200 mm/month.
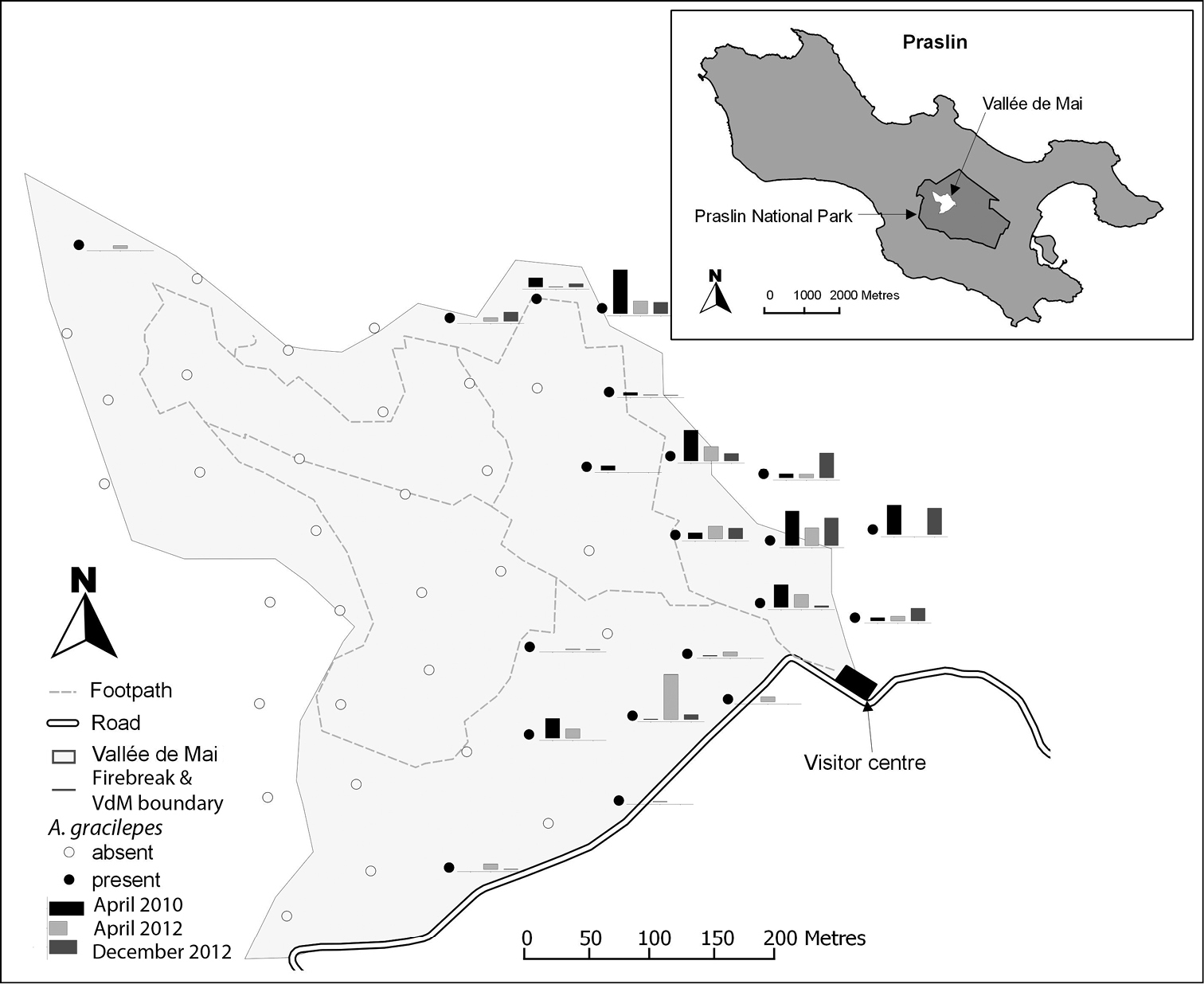
The distribution and abundance of Anoplolepis gracilipes in the Vallée de Mai, Seychelles. Circles indicate the sample locations and the presence (closed) or absence (open) of Anoplolepis gracilipes within 10 × 10 m quadrats. In April 2010 and December 2012 Anoplolepis gracilipes were observed at 14 locations, and in April 2012 ants were recorded from 18 locations in the Vallée de Mai. Bars next to full circles show relative Anoplolepis gracilipes activity in April 2010 (black), April 2012 (light grey) and December 2012 (dark grey), and are drawn to the same scale.
Anoplolepis gracilipes originates from either central east Africa or Asia but now has a pan-tropical and subtropical distribution. The species is a generalist and opportunistic consumer, which will predate and scavenge a variety of food sources. Workers make up >80% of the individuals in the nest and exhibit extensive foraging 24 h/day year round in areas where the climate does not hinder ant activity (
The main fieldwork was conducted over a 12-week period between March and June 2010. Fieldwork included surveys on ants and the arboreal endemic fauna and recordings of environmental variables. In addition, two ant distribution surveys were conducted in April 2012 (end of the wet season) and December 2012 (end of the dry season). To determine the distribution of Anoplolepis gracilipes, a grid of fifty 10×10 m quadrats (~2.5% of the total area) throughout the valley was surveyed. Quadrats were spaced 75×75 m apart and sampled along parallel N-S transects spanning the entire study area. Quadrats located in water or on large boulders were shifted to the nearest suitable adjacent area. Hereafter, the area of the Vallée de Mai with Anoplolepis gracilipes is referred to as ‘invaded’ and the area without Anoplolepis gracilipes as ‘uninvaded’.
We used ant activity counts to quantify Anoplolepis gracilipes abundance. We were not able to apply the more standard pitfall methods to assess Anoplolepis gracilipes abundance because the terrain of the Vallée de Mai consists of thick, multi-level palm leaf litter and boulders; therefore we adapted the method used by
At each ant count location we recorded canopy cover, ground surface temperature (recorded to 0.1 °C with a thermometer in the shade on the ground) and relative humidity (humidity meter ‘Rapitest’, Stanton Hope, Essex). Canopy cover was assessed by counting the number of quarters of a 10×4 cm tube that showed canopy when looking vertically upwards at each ant count location. A quarter was counted only if more than half of that quarter was covered by the canopy. Each counted quarter therefore represents a maximum of 25% canopy cover, i.e., 0 quarters = 0% canopy cover, 2 quarters = 50% canopy cover etc. Records from each ant count location were averaged to produce mean canopy cover, temperature and relative humidity per quadrat.
We also assessed canopy use by Anoplolepis gracilipes via tree trunks for each quadrat in the invaded area by searching trunks for one minute each and recording presence/absence of Anoplolepis gracilipes on five randomly selected adult trees in each invaded quadrat. No other ant activity was recorded on trunks in quadrats in either area.
Preliminary observations suggested that Anoplolepis gracilipes frequently used the palm forest canopy, which is dominated by large Lodoicea maldivica leaves. Because most of the endemic arboreal fauna of the Vallée de Mai is closely associated with Lodoicea maldivica, we expected any interference between Anoplolepis gracilipes and endemic arboreal species to occur predominantly on Lodoicea maldivica. We assessed the effect of Anoplolepis gracilipes presence by surveying eight species of arboreal endemic fauna likely to be directly affected by Anoplolepis gracilipes, which were recorded from all parts of the palm forest prior to the invasion of Anoplolepis gracilipes. The species surveyed were the day geckos Phelsuma astriata and Phelsuma sundbergi, the three species of bronze gecko Ailuronyx seychellensis, Ailuronyx tachyscopaeus and Ailuronyx trachygaster; and three arboreal molluscs Vaginula seychellensis, Stylodonta studeriana and Pachnodus pralines. The high density of Lodoicea maldivica in the Vallée de Mai and almost constant flowering of males provides a reliable food resource for these and other species. Surveys were made on 60 randomly selected trees (20 males, females and juveniles) of Lodoicea maldivica in each the invaded and uninvaded area. On each tree, a 5-min thorough search of the trunk, all stems, undersides of leaves and fruit/flowers was conducted with binoculars (magnification: 8×42) recording the number of individuals of each of the eight species.
We used a logistic regression model to test the influence of environmental variables on Anoplolepis gracilipes distribution. Dfbeta statistics, similar to Cook’s distance in linear models, is a measure of influence of individual points on logistic regression analysis (
In 2010, Anoplolepis gracilipes was confined to the north-east part of the Vallée de Mai, occurring in 14 of the 50 (28%) quadrats (Fig. 1). In April 2012, Anoplolepis gracilipes expanded its range to occupy 18 quadrats, including 12 of the 14 previously occupied, with the range expansion being from the firebreak in the east and north-east and from the road along the southern border. This was followed by, a range contraction in December 2012 to 14 previously occupied quadrats (Fig. 1). The activity of Anoplolepis gracilipes is lowest in the south-east and highest near the firebreak in the east close to the visitor centre, bordering the Vallée de Mai (Fig. 1). There was no change in mean ant activity (± SE) in the invaded area across surveys (2010: 3.55 ± 0.88 individuals/min-1; April 2012: 2.18 ± 0.58; December 2012: 2.57 ± 0.69; paired Wilcoxon test p > 0.1; range: 0.07 – 10.9). Mean Anoplolepis gracilipes activity in invaded quadrats at the edge of the Vallée de Mai (N = 6; 4.83 ± 0.94) was higher than those inside the forest (N = 8; 0.87 ± 0.31; Wilcoxon test p > 0.0036; Fig. 1) in December 2012, but not in 2010 and April 2012, suggesting a shift in ant abundance towards the firebreak in December 2012.
Invaded areas were characterised by higher humidity (invaded vs. uninvaded: 75.8 ± 0.8% vs. 72.9 ± 0.5% mean ± SE), lower canopy cover (2.5 ± 0.2% vs. 3.0 ± 0.1%), and slightly higher temperature (27.6 ± 0.13 °C vs. 27.5 ± 0.083 °C; Table 1), but variation of Anoplolepis gracilipes activity within invaded areas was not related to temperature (χ23, 10 = 0.04, p = 0.83), canopy cover (χ23, 10 = 1.21, p = 0.27) or humidity (χ23, 10 = 0.17, p = 0.69).
Logistic regression analysis showing the effect of environmental variables on the likelihood of Anoplolepis gracilipes presence or absence within quadrats in the Vallée de Mai (N = 47) (R2 = 0.63 (Nagelkerke), model χ2 = 26.16, classifies 94% correctly).
| Coefficient | SE | Wald χ2 | Odds ratio | 95% CI for Odds ratio | P | ||
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Temperature | 2.68 | 1.13 | 5.67 | 14.6 | 1.61 | 132 | 0.017 |
| Canopy cover | –2.36 | 0.96 | 6.05 | 0.094 | 0.014 | 0.619 | 0.014 |
| Humidity | 0.745 | 7.45 | 7.45 | 2.11 | 1.22 | 3.60 | 0.006 |
| Constant | –125 | 48.4 | 6.61 | 0.000 | 0.010 | ||
Anoplolepis gracilipes was observed primarily on the ground but was recorded on 35% (54 of 153) of trees in invaded quadrats, most commonly on introduced Cinnamomum verum (20%), followed by Lodoicea maldivica (13%). Overall, the relative abundance of introduced broadleaf trees was more than four times higher in the invaded (native:introduced = 6.75:1) than the uninvaded area (1.57:1). We opportunistically observed Anoplolepis gracilipes tending two species of honeydew-producing hemipterans, the soft scales Pulvinaria urbicola (Coccidae) and Icerya seychellarum (Margarodidae). Both species are introduced to the Seychelles and were tended on endemic and introduced dicotyledonous trees in and close to the firebreak. We randomly checked several hundred palm leaves during the study period and observed no tended hemipterans on any of the palm species.
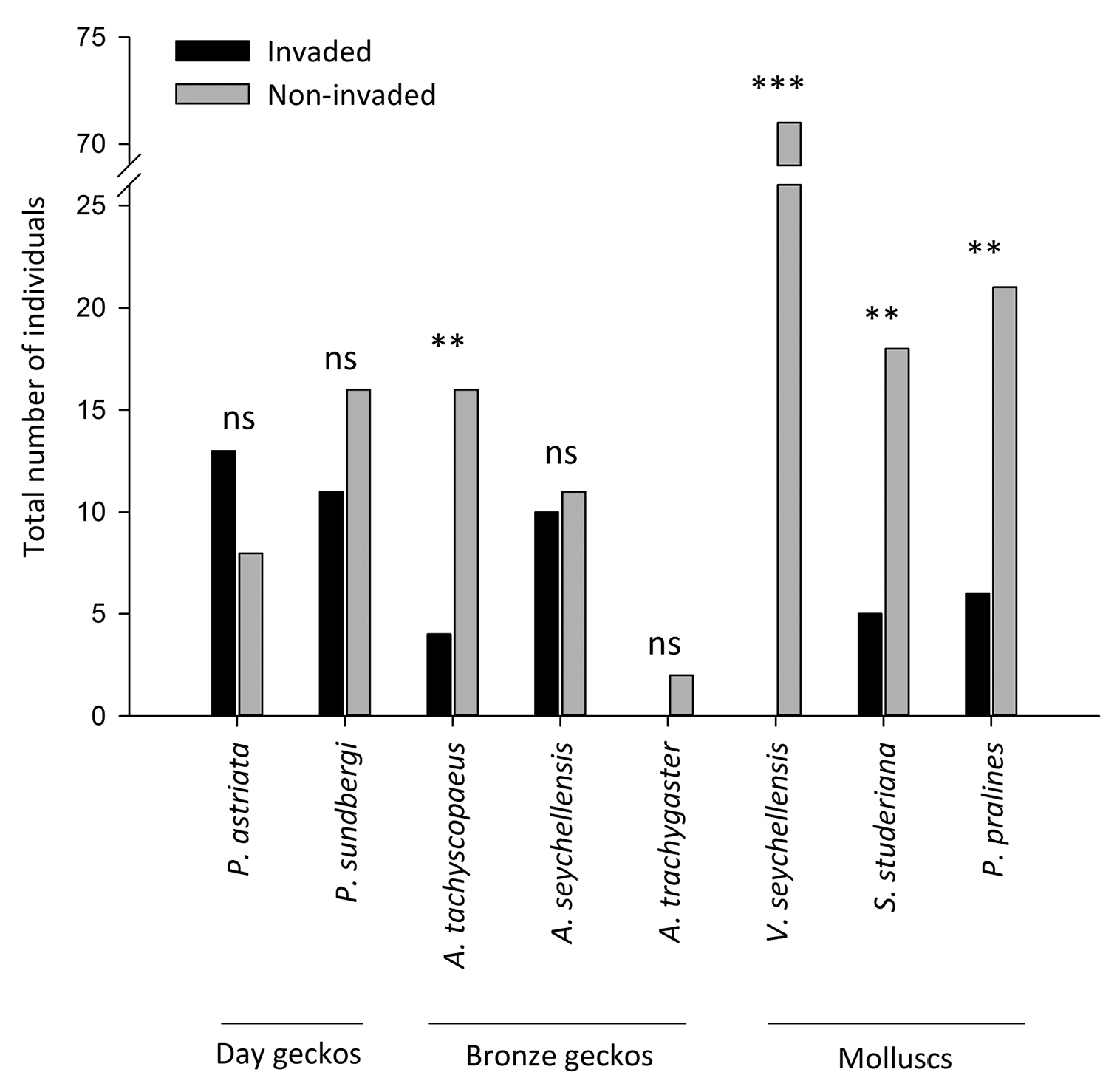
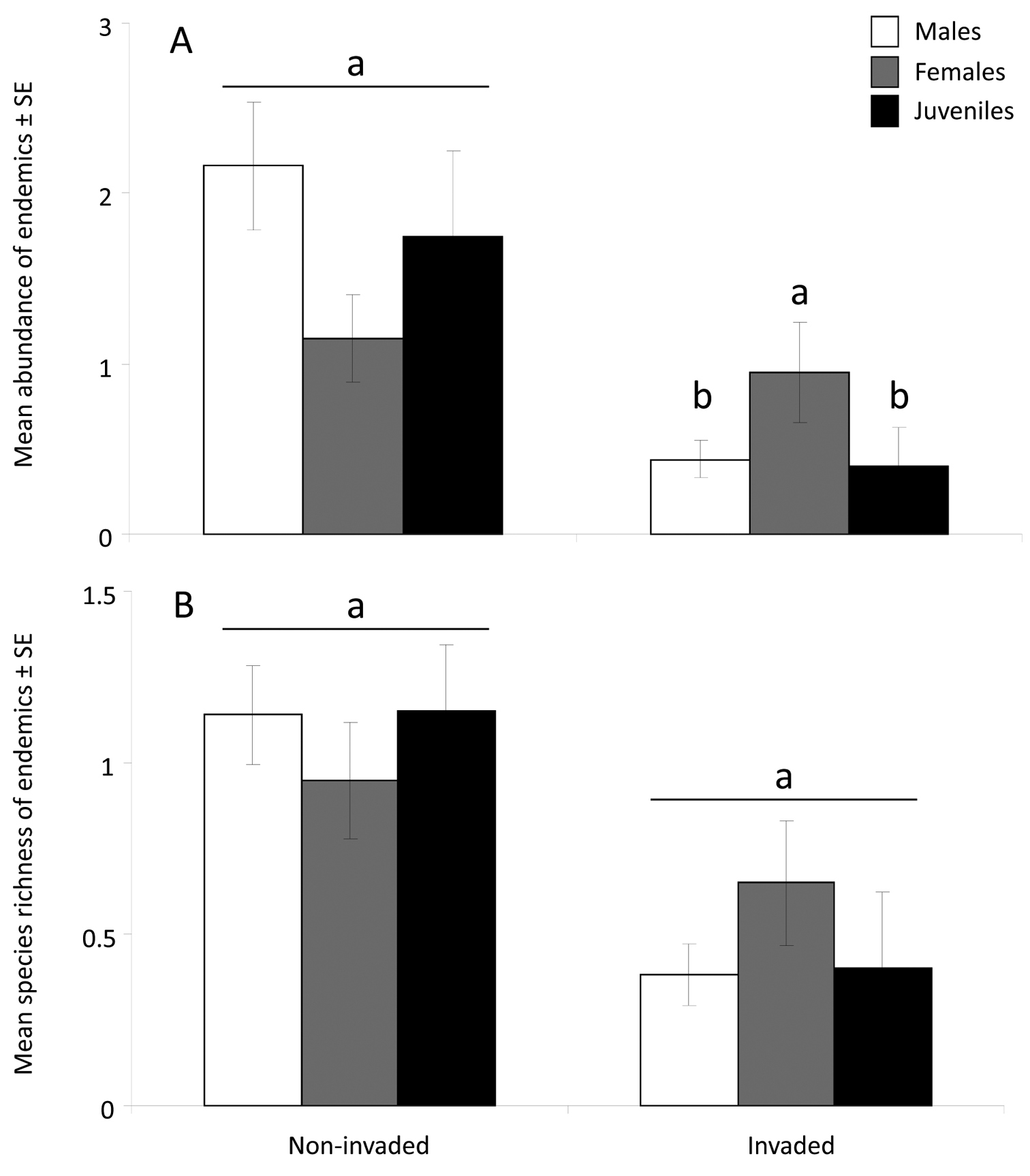
The endemic arboreal species displayed considerable variation between the invaded and uninvaded areas (Fig. 2). The molluscs Vaginula seychellensis, Stylodonta studeriana and Pachnodus pralines were less abundant or absent where ants occurred (χ2 = 69, p < 0.0001; χ2 = 9.8, p < 0.01; χ2 = 8.3, p < 0.01, respectively). The white slug Vaginula seychellensis was common in the uninvaded area but entirely absent from the invaded area. Of the geckos, only the dwarf bronze gecko Ailuronyx tachyscopaeus was significantly less abundant in the invaded area (χ2 = 7.2, p < 0.01). Abundance of the four species with the greatest differences between invaded and uninvaded areas showed no relationship with humidity or canopy cover (all r < 0.01, p > 0.05). Overall, mean species richness (invaded: 0.44 ± 0.08; uninvaded: 1.1 ± 0.10; χ25, 114 = 0.25, p = 0.002) and abundance (invaded = 46; uninvaded = 166; χ25, 114 = 17.01, p < 0.001) of endemic arboreal species on Lodoicea maldivica were lower in the invaded compared to uninvaded areas. There was no significant main effect for endemic species richness (χ25, 114 = 0.245, p = 0.89) and abundance (χ25, 114 = 0.614, p = 0.74) on male, female and juvenile Lodoicea maldivica within the invaded and uninvaded areas (Fig. 3). Although the abundance of endemic arboreal species on female Lodoicea maldivica trees was similar in Anoplolepis gracilipes invaded and non-invaded areas (p = 0.54), abundance on males and juveniles (p < 0.001) in the invaded area was significantly lower (area × tree type interaction effect; χ25, 114 = 6.50, p = 0.039; Fig. 3). There was no significant interaction effect between tree type and area in the number of endemic arboreal species (χ25, 114 = 2.73, p = 0.25).
Abundance of eight endemic species in the Anoplolepis gracilipes invaded and uninvaded areas: Number of observed individuals of eight species of endemic arboreal species on Lodoicea maldivica palms in the Anoplolepis gracilipes invaded (N = 60 trees) and uninvaded areas (N = 60 trees) within the Vallée de Mai. Counts were compared by chi-square test and levels of significance indicate: ns = non-significant, * < 0.05, ** < 0.01, *** < 0.001.
Differences in endemic species abundance and richness between the invaded and uninvaded areas and across tree classes.(A) Mean abundance and (B) species richness of endemic arboreal species in Anoplolepis gracilipes invaded and uninvaded areas are similar between 20 male, female and juvenile Lodoicea maldivica palms in the Vallée de Mai. Different small letters indicate significant differences in between-area comparisons (invaded vs. non-invaded) but not within-area comparisons. Full statistics are presented in the text.
Although Anoplolepis gracilipes has occurred across Praslin for at least the last decade and is present in Praslin National Park, there were no reports of the species occurring inside the Vallée de Mai until 2009. Despite its documented ability to rapidly cover large areas in high densities under optimal conditions (
Firstly, the occurrence of Anoplolepis gracilipes in the Vallée de Mai may be due to localised introduction and disturbance close to the palm forest. Repeated introductions and anthropogenic disruption of ecosystems both increase the likelihood of successful species invasions (
Secondly, the lack of population growth in the Vallée de Mai may be due to a shortage of liquid sugary substances, which is an important energy source for workers, especially during invasions (
Thirdly, biotic resistance may also explain the observed stable population of Anoplolepis gracilipes in the Vallée de Mai. There is little and ambiguous information on the role of native ant species in conferring biotic resistance to the invasion of exotic ant species. Both
A final explanation for the apparent dynamic equilibrium of the Anoplolepis gracilipes population concerns abiotic conditions.
We observed marked variation between the presence of Anoplolepis gracilipes and several arboreal species. The number and abundance of endemic arboreal species was lower on Lodoicea maldivica in invaded areas, and the effect was particularly strong for molluscs, which were abundant throughout the palm forest prior to the arrival of Anoplolepis gracilipes (NB, pers. obs.). To our knowledge this is the first record of Anoplolepis gracilipes invasion coinciding with the disappearance of native molluscs, although cause and effect could not be confirmed. Further spread of Anoplolepis gracilipes through the palm forest could threaten the viability of these species and the relationship between molluscs and palms in this forest. The absence of the slug Vaginula seychellensis, a Lodoicea maldivica specialist, from the invaded area is particularly concerning. Effects of Anoplolepis gracilipes on native species were also recorded on Mahé, where fewer terrestrial reptiles occurred in Anoplolepis gracilipes invaded areas (
Island endemics are typically at higher risk of extinction (
We thank Wilna Accouche, Marc Jean-Baptiste, Frauke Fleischer-Dogley and Uzice Samedi of the Seychelles Islands Foundation for project support and help with fieldwork, and the Seychelles Bureau of Standards and the Department of Environment for approving the research. We are grateful to Nico Blüthgen, Lori Lach, and three anonymous reviewers for insightful comments. Brian Fisher kindly provided the ant reference collection for species identification in the Vallée de Mai. The European Union provided funding for part of the study under the project “Mainstreaming the management of invasive species as fundamental to preserving the ecological integrity and enhancing the resilience of Seychelles’ World Heritage Sites” (DCI-ENV/2010/220-252), and CKB acknowledges funding from the German Research Foundation (KA 3349/2-1).