Citation: Thomsen MS, Wernberg T, Olden JD, Byers JE, Bruno JF, Silliman BR, Schiel DR (2014) Forty years of experiments on aquatic invasive species: are study biases limiting our understanding of impacts? NeoBiota 22: 1–22. doi: 10.3897/neobiota.22.6224
Invasions by non-native species are a threat to biodiversity because invaders can impact native populations, communities and entire ecosystems. To manage this threat, it is necessary to have a strong mechanistic understanding of how non-native species affect local species and communities. We reviewed 259 published papers (1972–2012) that described field experiments quantifying the impact of aquatic non-native species, to examine whether various types of study biases are limiting this understanding. Our review revealed that invasion impacts had been experimentally quantified for 101 aquatic non-native species, in all major freshwater and marine habitats, on all continents except Antarctica and for most higher taxonomic groupings. Over one-quarter (26%) of studies included tests for impacts on local biodiversity. However, despite this extensive research effort, certain taxa, habitats and regions remain poorly studied. For example, of the over one hundred species examined in previous studies, only one was a marine fish and only six were herbivores. Furthermore, over half (53%) of the studies were from the USA and two-thirds (66%) were from experiments conducted in temperate latitudes. By contrast, only 3% of studies were from Africa and <2% from high latitudes. We also found that one-fifth (20%) of studies were conducted in estuaries, but only 1% from coral reefs. Finally, we note that the standard procedure of pooling or not reporting non-significant treatments and responses is likely to limit future synthetic advancement by biasing meta-analysis and severely limiting our ability to identify non-native species with none or negligible ecological impacts. In conclusion, a future focus on poorly-studied taxa, habitats and regions, and enhanced reporting of results, should improve our understanding and management of impacts associated with aquatic non-native species.
Biotic homogenization, alien species, exotic species, review
Invasion by non-native species can alter community structure and ecosystem functions with significant impacts on biodiversity (
The discipline of invasion ecology has grown immensely in recent decades with papers published across an increasing number of general scientific (ecology, invasion biology, conservation biology) and subject-specific (terrestrial, freshwater and marine) journals. Recently,
Our objectives were to quantify the breadth of field-based experimental studies on invasion impacts in aquatic ecosystems, to identify what species, regions and environments have been targeted in these tests, and whether there have been any general problems relating to their experimental design and data reporting. Analogue reviews have provided important knowledge about research biases in other fields, including climate change, terrestrial ecology, conservation biology and general invasion biology (
To achieve our objectives, we reviewed field-based experiments on invasion impacts to identify what species, regions and environments have been targeted in these tests, and whether there have been any general problems relating to their experimental design and data reporting. We hope that this review will stimulate experimental research about aquatic invasion impact and this will lead to the development of new predictive tools in conservation management.
We searched for peer-reviewed, manipulative aquatic field experiments in Web of Science, Current Contents, and Google Scholar, using various combinations of ‘impact’, ‘effect’, ‘non-native’, ‘non-indigenous’, ‘exotic’, ‘invader’, ‘alien’, ‘aquatic’, ‘marine’, and ‘freshwater’. Reference lists were back-tracked from existing review papers and frequently cited impact studies (e.g.
First, we evaluated the published research in relation to the attributes of individual studies. We categorized whether the journal in which experiments were published targeted general scientists, freshwater, marine, or aquatic biologists (see Suppl. material 1 for detail). We recorded the year of publication and determined whether the experimental design was spatially pseudo-replicated (i.e. with only one ‘spatially independent’ control and/or treatment,
Second, we evaluated study characteristics related to the attributes of the non-native species, that is whether the non-native species was a plant or animal, pre-dominantly occupied the pelagic or benthic realm, was mobile or sessile, whether its trophic position was a plant, filter-feeder, herbivore, omnivore or carnivore, and its taxonomic affiliation. We classified very slow-moving bivalves as sessile taxa, and grouped consumers of macroscopic or microscopic primary producers (the latter group includes sediment eaters and detrivores) together to represent herbivores.
Third, we evaluated study characteristics related to the attributes of the invaded system. Here we extracted data about the spatial location of each experiment. Studies that included nested designs or multiple experiments, where study sites were separated by >1 km, were added as independent locations. These data were also grouped into invaded continent and latitude (proxy for climate). We then noted if the invaded system was a freshwater or marine habitat, where estuaries and salt marshes were grouped as marine. This attribute represents the invaded system (instead of the non-native species) because the same non-native species (e.g., the common reed Phragmites australis) can impact both marine and freshwater habitats. These two broad habitat types were subdivided into streams, ponds, lakes, wetlands, estuaries, open coast marine sands, rocky intertidal, or rocky subtidal, where wetlands included a few riparian studies, ponds included experiments conducted in outdoor freshwater mesocosms (ponds and mesocosm experiments are both conducted in relatively small enclosed systems), estuaries included both intertidal and subtidal studies, and the rocky subtidal zone included a few coral reef experiments (too few to justify a separate analysis for this habitat, see discussion for detail).
Finally, we examined if taxa were studied in proportion to their ‘recognized occurrence as non-native species’. We used
Our initial search suggested that more than 700 aquatic invasion impact studies have been published. However, after excluding reviews (
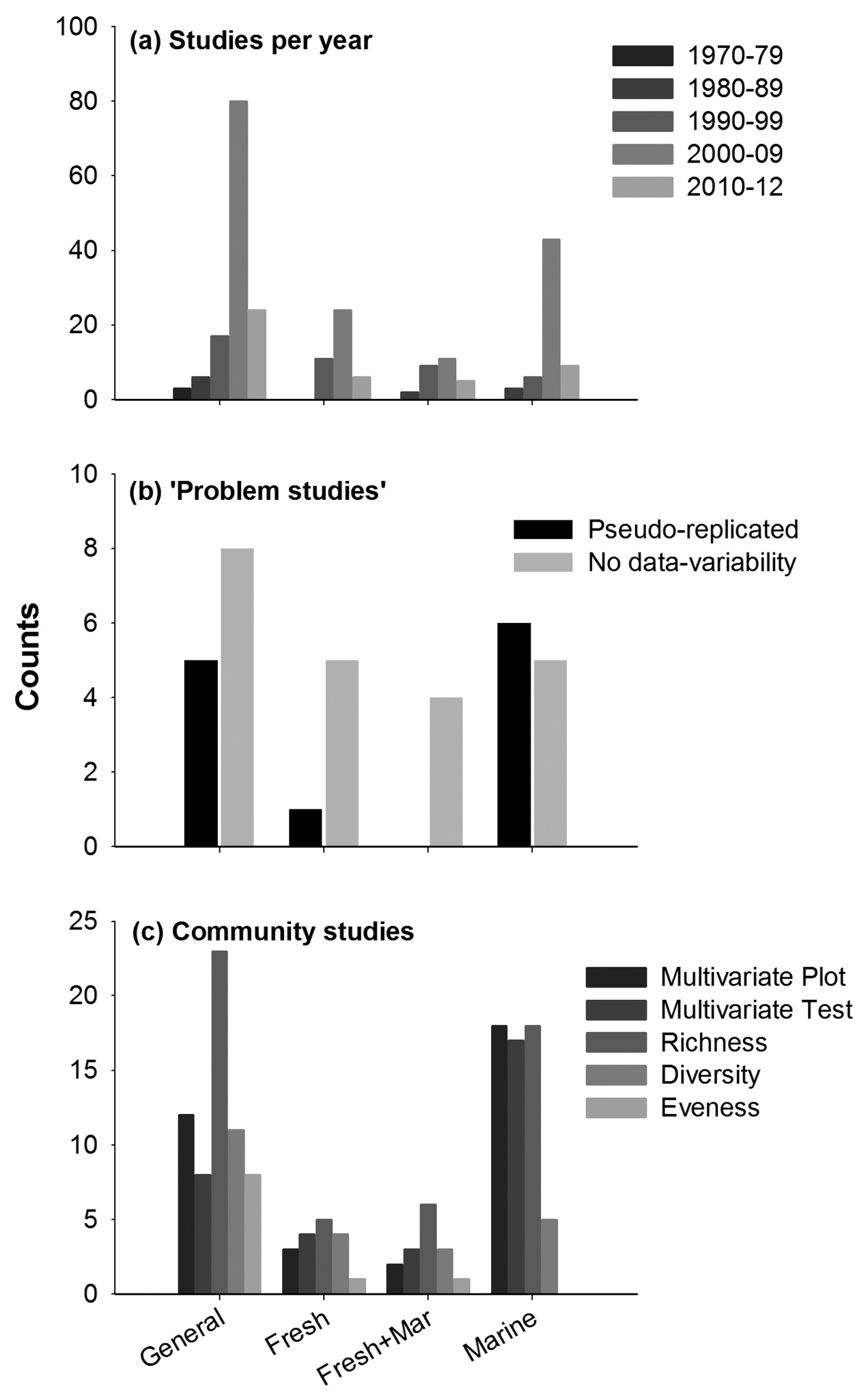
Evaluating research in relation to study attributes revealed that many more papers were published in journals targeting general readers (130) than marine (61), freshwater (41) or freshwater and marine (27) readers. Publications of invasion impact papers increased rapidly for all journal types (Fig. 1a). The first study was published in
Aquatic invasion impact experiments published in general, freshwater, marine, and both freshwater and marine journals (see S1 for journal classification) a decadal trend in publication rates (the last bar only include studies published 2010–12) b Studies where the experimental design was spatially pseudo-replicated or did not replicate treatment or control plots, and studies that did not report any measure of variability for impact data c Community impact studies that quantified impact with graphical display, multivariate inferential tests, or on taxonomic richness, diversity or evenness.
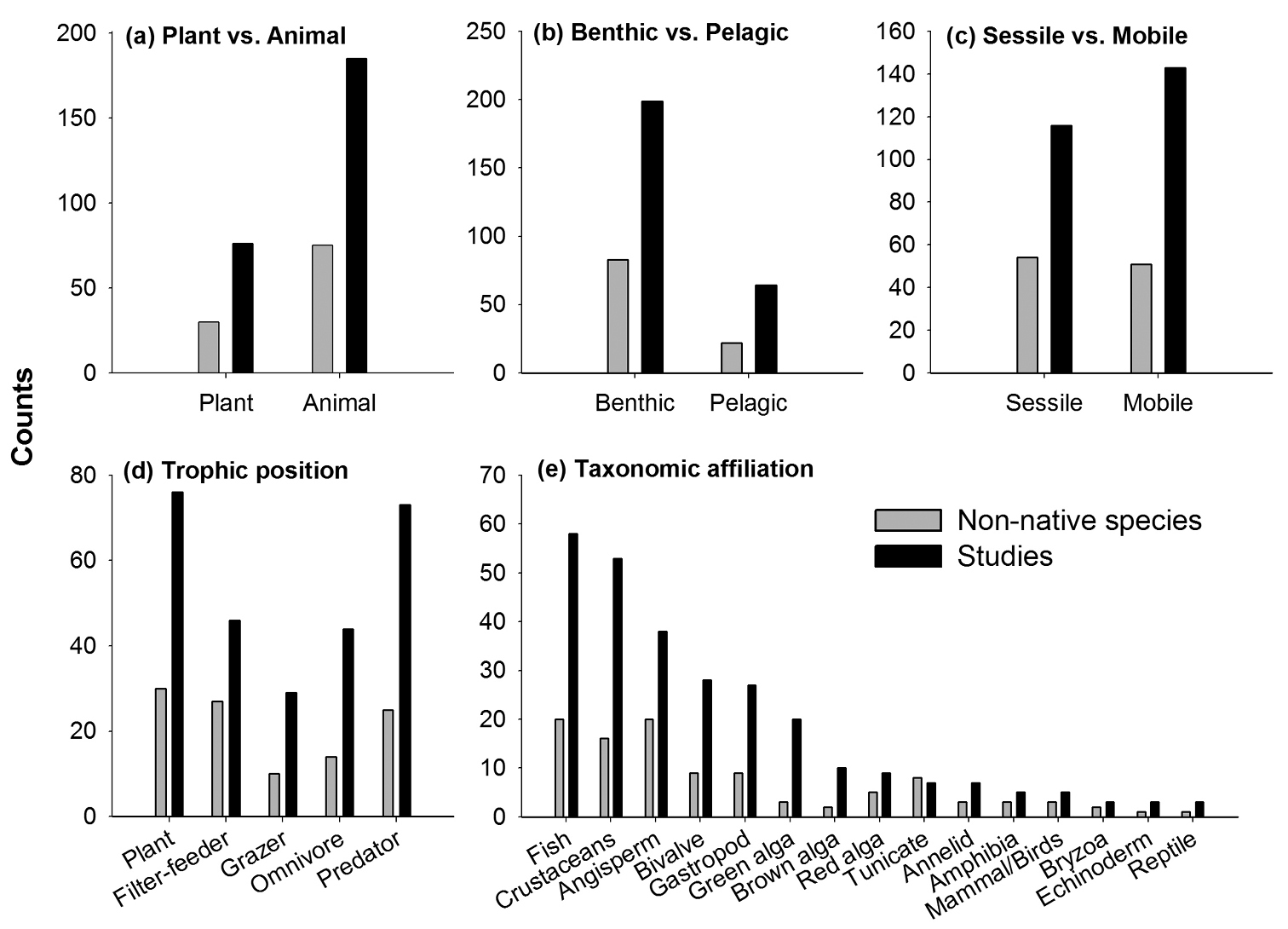
Furthermore, when we evaluated study characteristics in relation to invader attributes we found that more experiments have been done on non-native animals (75 species, 185 studies) than plants (30, 76) (Fig. 2a), on benthic (83, 199) than pelagic (22, 64) invaders (Fig. 2b), and on almost the same number of sessile (54, 116) and mobile (51, 143) invaders (Fig. 2c). Subdividing non-native animals into trophic position showed that carnivores (25, 73) were more studied than filter-feeders (27, 46) and omnivores (14, 44), and that few invasive herbivores and detrivores have been studied (10, 29) (Fig. 2d). Of these 10 species, 6 were herbivores with a capacity to denude large vegetation (the mammal Myocastor coypus, the bird Cygnos olor and the four snails Littorina littorea, Bellamya chinensis, Physella acuta, Pomacea canaliculata), whereas the remaining 4 non-native species were sediment-eaters and detrivorous snails and crustaceans (Batillaria attramentaria, Ilyanassa obsoleta, Potamopyrgus antipodarum, Limnomysis benedeni). The most frequent experiments were on non-native fish (20 species, 58 studies - where two studies were from marine systems and the lionfish Pterois volitans), vascular plants (20, 38) molluscs (18, 55), crustaceans (16, 53) and algae (10, 39). By contrast, relatively few experiments were on non-native tunicates (8 species, 7 studies), annelids (3, 7), amphibians (3, 5), mammals and birds (3, 5), bryozoans (2, 3), echinoderms (1, 3) or reptiles (1, 3) (Fig. 2e).
Aquatic invasion impact experiments classified by the attributes of the non-native species. Grey bars correspond to number of invasive species studied (total of 105 taxa); black bars correspond to the number of scientific studies (total of 259 studies). Non-native species were classified as a being a plant or animal b if they occupy the benthic or pelagic realm c if they are sessile or mobile d according to their trophic position (mud-eaters were classified as herbivores, as they consume diatoms), and e taxonomic affiliation.
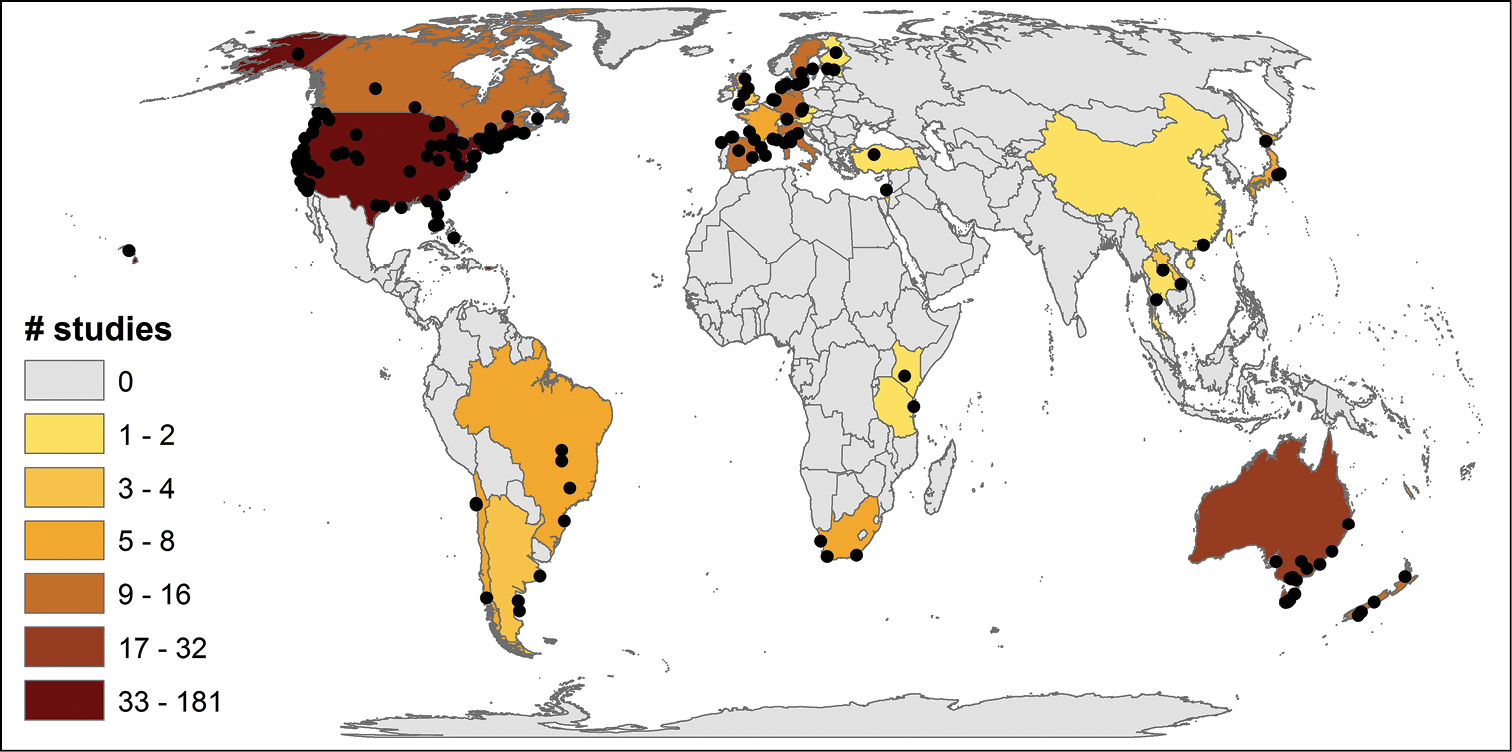
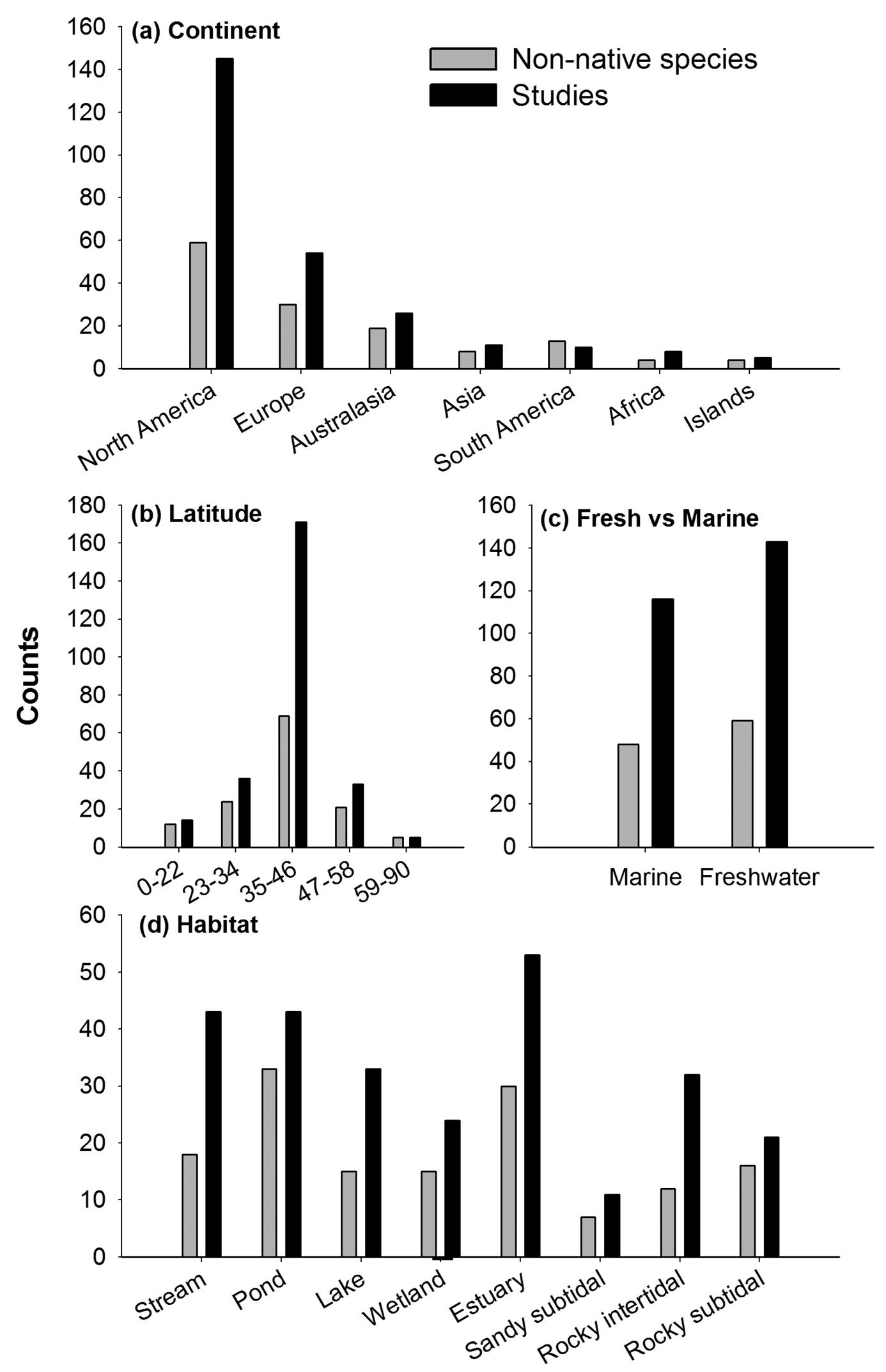
Evaluating study characteristics in relation to the invaded system attributes showed a strong geographical pattern (Fig. 3); impacts documented in the 259 papers represent studies from 30 countries and were strongly dominated by the USA (60 species; 137 studies). Furthermore, only 9 other countries have tested for impacts of ≥5 non-native species, including Australia (13; 21), Germany (8; 7), Brazil (6; 4), Canada (8; 11), Japan (6; 5), New Zealand (6, 5), Italy (5; 12), Spain (5; 10), and France (5; 4). This geographical pattern was also evident when grouped into continental scales; invasion impacts have been tested mainly in North America (59 species, 145 studies), Europe (30; 54), and Australasia (19; 26). Far fewer non-native species have been tested from South America (13; 10), Asia (8; 11), Africa (4; 8) and various islands (4; 5) (Fig. 3, 4a).
Global distribution of field based aquatic invasion impact experiments (n = 301; 259 reviewed studies + 42 extra locations from nested or multiple spatially separated experiments within studies).
Aquatic invasion impact experiments classified by the attributes of the invaded system. Grey bars correspond to number of non-native species studied out of 105 taxa, and black bars correspond to the number of scientific studies out of 259. The invaded system was classified according to a continent b latitude c if it was a marine or freshwater system, and d habitat.
We also found a strong geographical pattern along a latitudinal (climate-related) gradient; most studies were from the mid-latitudes of 35–46° (69 species, 171 studies), followed by 23–34° (24; 36) and 47–58° (21; 33). By contrast, few non-native species have been studied from tropical or cold/polar regions (0–22° = 12 species, 14 studies; 59–90° = 5 species, 5 studies – and these studies were all from 59–64° N) (Fig. 3, 4b). Finally, we found that more studies have been conducted in freshwater (59 species, 143 studies) than marine (48; 116) habitats (Fig. 4c), or more specifically, that most aquatic invasive species have been studied in ponds (33; 43, including mesocosm experiments), followed by estuaries (30; 53), streams (18; 43), wetlands (15; 24), rocky subtidal (16; 21, including 3 species and 4 studies from coral reefs), lakes (15; 33), rocky intertidal (12; 32), and sandy open subtidal habitats (7; 11) (Fig. 4d).
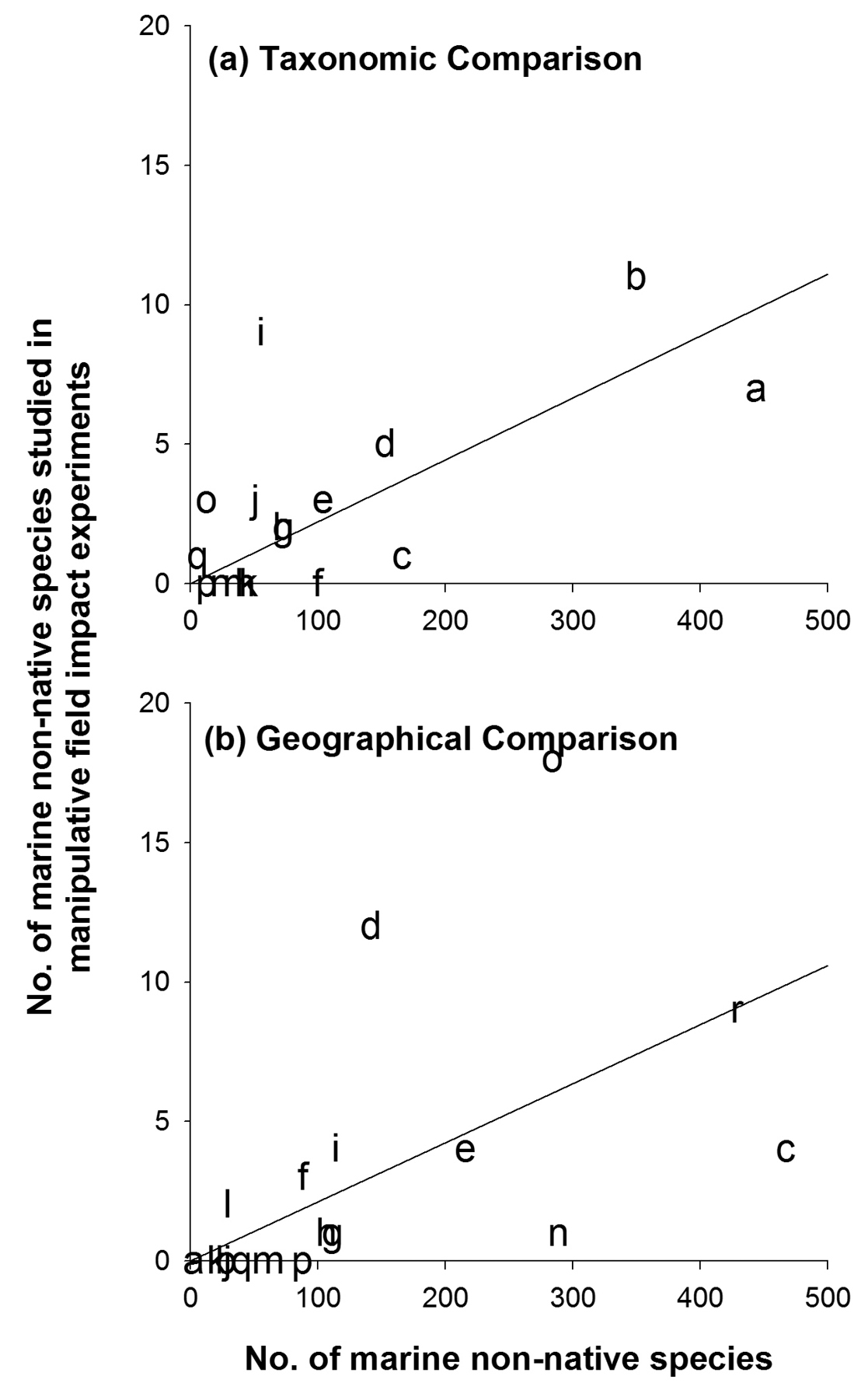
Finally, when we compared our data to Hewitt & Campbell’s compilation of marine non-native and cryptogenic species we found a significant slope, indicating that the number of marine species grouped into different invasive taxa (Fig. 5c; slope = 0.022, p = 0.0001, n = 17) and invaded biogeographical regions (Fig. 5d; slope = 0.021, p = 0.0006, n = 18) correlated with number of taxa and regions tested in manipulative field experiments. However, even though the line fit well generally, there were still over and under-represented taxa; for example, experimental studies on molluscs, angiosperms and chordates (mainly tunicates) were over-represented (well above the line), whereas fish, crustaceans and cnidarians were under-represented (well below the line). Similarly, experimental studies from the North East Pacific and the North West Atlantic were over-represented, whereas experimental studies from the Southern Pacific Ocean and the Mediterranean Sea were under-represented. Both regression slopes were small, showing that ca. 2 non-native species had been tested in field impact-experiments for every 100 known non-native marine species.
Marine invasion impact experiments classified according to a taxonomic identity of the non-native species and b invaded biogeographical regions. Graphs depict linear regression between manipulative marine field-based invasion impact studies against the total number of non-native and cryptogenic marine species, see manuscript text for regression data. Abbreviations (following
Virtually all the aquatic invasion examples presented in Charles Elton’s seminal book “The Ecology of Invasions by Animals and Plants” were based on anecdotal or observational data (
Attributes of individual studies. We found an exponential increase in experimental impact studies over the past few decades. If this trend continues, >500 experimental aquatic impact studies could be published in the next decade. It is vital that this (expensive) effort builds on previous research rather than repeats it; i.e., new experiments should be conducted in a context of the listed studies in the Suppl. material 1. For example, c. 5% of reviewed studies were spatially pseudo-replicated, a design that should be avoided whenever possible (although in some cases this may be impossible, especially for broad-scale studies) (
We also noted that it was common practice to pool non-significant treatments, to not report non-significant effects, or to report only a subset of quantified responses. If future studies continue to value significance over effect size, for example only reporting significant results (the extreme case being the unknown number of studies that are never published because there are no significant results), the focus will remain on “high-impact invaders” only, thereby limiting our ability to understand contextual differences between high and low invasion impact (
Our literature list indicates that aquatic impact studies may have been overlooked in invasion biology. As stated in the introduction, reviews of plant invasion impacts have not included examples of impacts of seaweeds or seagrasses (cf. reviewed reference listings,
Attributes of the non-native species. More than twice as many non-native animal species than plants have been tested in field impact experiments, most likely reflecting the fact that more non-native animals than plants have been found (
Analysis across trophic levels revealed that non-native herbivores have been poorly studied. Studies are clearly needed on more aquatic herbivores to better understand, predict and manage their impacts on aquatic plant communities (
Only 2% of recently tallied marine non-native and cryptogenic species have been tested in field impact experiments, demonstrating that basic mechanistic insight on impact is lacking for most marine non-native species. This number varied with taxonomic groups from 0% (e.g. cnidarians, porifera) to 22% (angiosperms). We found few experimental impact studies conducted on non-native cnidarians, porifera, annelids, bryozoans, echinoderms, amphibians, mammals, or reptiles, reflecting in part, that there are few species in these groups (at least for marine organisms, Fig. 5,
Attributes of the invaded system. Not surprisingly, we found strong geographical patterns; many more aquatic invasion impact studies have been done in North America than in other countries or continents. Analysing biogeographical patterns in more detail for marine non-native species revealed a similar pattern; studies from the NE Pacific and NW Atlantic were over-represented (Fig. 4d) compared to most other marine regions. Scientific over-representation from North America, Europe and Australasia, has been documented across ecological sciences, including research on climate changes (
We also noted that invasion impacts have been tested with few non-native species for the majority of countries, a typical spatial unit with a specific set of management rules and regulations for invasive species. Given that invasion impacts can depend strongly on the local and regional context (
Research gaps associated with invaded habitat attributes were less pronounced compared to the geographical patterns, as also noted more generally for all types of invasion studies (
Over the past four decades, invasion impacts have been quantified in field experiments for more than 100 aquatic non-native species published in at least 259 peer-reviewed papers. However, despite this intensive research, our ability to make generalizations and predictions remain limited because impact depends on the specific context that links attributes of the non-native species and the invaded system. Regardless of the large experimental effort, our review revealed substantial gaps in the collective knowledge. Specifically, of the 101 test species, only one was a marine fish and only six were herbivores. Similarly, of the 259 papers, only 3% were from Africa, <2% from high latitudes and only 1% from coral reefs. We therefore recommend that future experiments target these less studied non-native species, regions and habitats. We also noted that it is standard to pool or not report non-significant treatments and responses, a procedure that limits synthetic advancement by biasing meta-analysis and by making it difficult to identify invaders and environmental conditions that result in weak impacts. We therefore also recommend to report non-significant and low impact data with associated data-variability (or for continuous design show scatter-plots with explicit identification of overlapping points) and to report non-pooled impact data for all treatments and responses (even if just in online appendixes). We suggest that our ability to extrapolate impact assessments across space, time and taxa will increase significantly if these research gaps are targeted. More generally, we argue that impact experiments should manipulate new and novel combinations of different non-native species, different invaded places, different resident organisms, different abiotic conditions and different resource levels, than has already been tested in past experiments (cf. Suppl. material 1). In conclusion, the last 40 years of research activity has provided an excellent starting point to understand invasion impact mechanistically, but we are still far from being able to build generalized and predictive models of invasion impacts on local management scales of most invaders.
TW was funded by the Australian Research Council. We thank the New Zealand Ministry of Science and Innovation and the National Institute of Water and Atmospheric Research for continued support of DRS.
List of reviewed references.
Authors: Mads S. Thomsen, Thomas Wernberg, Julian D. Olden, James E. Byers, John F. Bruno, Brian R. Silliman & David R. Schiel
Data type: reference list
Explanation note: References are divided into general (G), freshwater (F), aquatic (FM) or marine (M) journals. References with an asterisk (*) did not replicate or pseudo-replicated either treatment and/or control plots.
Copyright notice: This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.