Research Article |
|
Corresponding author: Alain Roques ( alain.roques@inra.fr ) Corresponding author: You-qing Luo ( youqingluo@126.com ) Academic editor: Marc Kenis
© 2023 Alain Roques, Lili Ren, Davide Rassati, Juan Shi, Evgueni Akulov, Neil Audsley, Marie-Anne Auger-Rozenberg, Dimitrios Avtzis, Andrea Battisti, Richard Bellanger, Alexis Bernard, Iris Bernadinelli, Manuela Branco, Giacomo Cavaletto, Christian Cocquempot, Mario Contarini, Béatrice Courtial, Claudine Courtin, Olivier Denux, Miloň Dvořák, Jian-ting Fan, Nina Feddern, Joseph Francese, Emily K. L. Franzen, André Garcia, Georgi Georgiev, Margarita Georgieva, Federica Giarruzzo, Martin Gossner, Louis Gross, Daniele Guarneri, Gernot Hoch, Doris Hölling, Mats Jonsell, Natalia Kirichenko, Antoon Loomans, You-qing Luo, Deborah McCullough, Craig Maddox, Emmanuelle Magnoux, Matteo Marchioro, Petr Martinek, Hugo Mas, Bruno Mériguet, Yong-zhi Pan, Régis Phélut, Patrick Pineau, Ann M. Ray, Olivier Roques, Marie-Cécile Ruiz, Victor Sarto i Monteys, Stefano Speranza, Jiang-hua Sun, Jon D. Sweeney, Julien Touroult, Lionel Valladares, Loïs Veillat, Yuan Yuan, Myron P. Zalucki, Yunfan Zou, Alenka Žunič-Kosi, Lawrence M. Hanks, Jocelyn G. Millar.
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation:
Roques A, Ren L, Rassati D, Shi J, Akulov E, Audsley N, Auger-Rozenberg M-A, Avtzis D, Battisti A, Bellanger R, Bernard A, Bernadinelli I, Branco M, Cavaletto G, Cocquempot C, Contarini M, Courtial B, Courtin C, Denux O, Dvořák M, Fan J-t, Feddern N, Francese J, Franzen EKL, Garcia A, Georgiev G, Georgieva M, Giarruzzo F, Gossner M, Gross L, Guarneri D, Hoch G, Hölling D, Jonsell M, Kirichenko N, Loomans A, Luo Y-q, McCullough D, Maddox C, Magnoux E, Marchioro M, Martinek P, Mas H, Mériguet B, Pan Y-z, Phélut R, Pineau P, Ray AM, Roques O, Ruiz M-C, Sarto i Monteys V, Speranza S, Sun J-h, Sweeney JD, Touroult J, Valladares L, Veillat L, Yuan Y, Zalucki MP, Zou Y, Žunič-Kosi A, Hanks LM, Millar JG (2023) Worldwide tests of generic attractants, a promising tool for early detection of non-native cerambycid species. In: Jactel H, Orazio C, Robinet C, Douma JC, Santini A, Battisti A, Branco M, Seehausen L, Kenis M (Eds) Conceptual and technical innovations to better manage invasions of alien pests and pathogens in forests. NeoBiota 84: 169-209. https://doi.org/10.3897/neobiota.84.91096
|
Abstract
A large proportion of the insects which have invaded new regions and countries are emerging species, being found for the first time outside their native range. Being able to detect such species upon arrival at ports of entry before they establish in non-native countries is an urgent challenge. The deployment of traps baited with broad-spectrum semiochemical lures at ports-of-entry and other high-risk sites could be one such early detection tool. Rapid progress in the identification of semiochemicals for cerambycid beetles during the last 15 years has revealed that aggregation-sex pheromones and sex pheromones are often conserved at global levels for genera, tribes or subfamilies of the Cerambycidae. This possibly allows the development of generic attractants which attract multiple species simultaneously, especially when such pheromones are combined into blends. Here, we present the results of a worldwide field trial programme conducted during 2018–2021, using traps baited with a standardised 8-pheromone blend, usually complemented with plant volatiles. A total of 1308 traps were deployed at 302 sites covering simultaneously or sequentially 13 European countries, 10 Chinese provinces and some regions of the USA, Canada, Australia, Russia (Siberia) and the Caribbean (Martinique). We intended to test the following hypotheses: 1) if a species is regularly trapped in significant numbers by the blend on a continent, it increases the probability that it can be detected when it arrives in other countries/continents and 2) if the blend exerts an effective, generic attraction to multiple species, it is likely that previously unknown and unexpected species can be captured due to the high degree of conservation of pheromone structures within related taxa. A total of 78,321 longhorned beetles were trapped, representing 376 species from eight subfamilies, with 84 species captured in numbers greater than 50 individuals. Captures comprised 60 tribes, with 10 tribes including more than nine species trapped on different continents. Some invasive species were captured in both the native and invaded continents. This demonstrates the potential of multipheromone lures as effective tools for the detection of ‘unexpected’ cerambycid invaders, accidentally translocated outside their native ranges. Adding new pheromones with analogous well-conserved motifs is discussed, as well as the limitations of using such blends, especially for some cerambycid taxa which may be more attracted by the trap colour or other characteristics rather than to the chemical blend.
Keywords
Cerambycidae, early detection, Holarctic, invasion, multi-pheromone blend, pheromone trapping
Introduction
During the last several decades, the unprecedented development of worldwide trade has resulted in increasing translocation and establishment of non-native insects outside their native ranges, with little evidence of saturation (
Another key attribute of this recently-arrived, non-native entomofauna is the increasing presence of “emerging” species, which have not been reported previously as invaders and are not considered to be pests in their native ranges. Arrival of these species probably results from evolving changes in trade routes and imported goods, which leads to accessibility to new pools of species (
Deployment of traps baited with broad-spectrum semiochemical lures at ports-of-entry (
This large family of Coleoptera includes between 34,000 and 38,000 described species (
Recent advances in the chemical ecology of cerambycids and, particularly, the identification of volatile pheromones that act as long-range attractants, have provided new tools and opportunities for monitoring invasive woodborers. In total, pheromones or likely pheromones have been identified for more than 400 cerambycid species worldwide (
During the last 10 years, the generic effectiveness of such multi-component blends has been tested on different continents, but using different pheromone combinations, either alone or in combination with kairomones, such as ethanol and α-pinene (e.g.
When using multi-pheromone blends, antagonistic effects might occur with either pheromone components or host plant volatiles (e.g.
Results of these different experiments on various continents stimulated us to propose a worldwide trapping programme using a standardised ‘generic’ 8-pheromone blend in all countries/trapping sites. The blend included the following compounds known to be widely shared amongst cerambycids of related taxa: fuscumol, fuscumol acetate, monochamol, geranylacetone, anti-2,3-hexanediol, 3-hydroxyhexan-2-one (C6-ketol), 2-methylbutan-1-ol and prionic acid. The programme relied on the following hypotheses: 1) if a species is attracted in significant numbers by the blend in a region, it increases the probability that it can be detected when it arrives at ports-of-entry in other regions and 2) if the blend exerts an effective, generic attraction to multiple species, it is likely that previously unknown and unexpected species can be captured due to the high degree of conservation of pheromone structures within related taxa, as described above. Our overarching objective was to build a global database of cerambycid species trapped by the 8-pheromone blend. To this end, field trials were conducted during 2018–2021 using operational protocols that were standardised as much as possible at all sites worldwide to cover simultaneously or sequentially 13 European countries, 10 Chinese provinces and some regions of the USA, Canada, Australia, Russia (Siberia) and the Caribbean. Over the course of the study, we also tested the possibility of adding new compounds to enlarge the pool of species trapped. Therefore, in 2020, two additional pheromones, the sex-aggregation pheromones trichoferone (a hydroxyketone pheromone of the velvet longhorned beetle, Trichoferus campestris (Faldermann) (
Materials and methods
Study sites
The successive or parallel development of three European research projects (HOMED, MULTITRAP, SAMFIX) and two French projects (CANOPEE, PORTRAP) during 2018–2021 allowed us to carry out field trials at 302 sites distributed as follows: 244 in Europe (164 in France, 22 in Italy, 13 in Spain and Switzerland, 6 in Portugal, 5 in Austria and England, 4 in Greece and Slovenia, 3 in the Netherlands, 2 in Bulgaria and the Czech Republic and 1 in Sweden), 38 in Asia (35 in China and three in Siberia, Russia), 11 in North America (10 in the USA and one in Canada), five in the Caribbean (Martinique) and four in Australia (see Table
Summary of the trapping design per country from 2018 to 2021. Research project: C: CANOPEE; H: HOMED; M: MULTITRAP; P: PORTRAP; S: SAMFIX. Blend: #8: 8-pheromone blend; #8+ET: 8-pheromone blend + ethanol UHR; #8+AP+ ET: 8-pheromone blend + α-pinene + ethanol UHR; #10+ AP+ET: 10- pheromone blend + α-pinene + ethanol UHR. Trap type: MF: multifunnel; CV: crossvane. Trap colour: B: black; G: Green; BG: Black base and green top; P: Purple; Y: Fluorescent yellow; others: brown, blue, red, grey (corresponding to data collected by
| Region | Year | Country/ Province | Project | No Sites | No Traps | Blend | Trap type | Trap color | Collection Type | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| #8 | #8+ET | #8+AP+ET | #10+AP+ET | MF | CV | B | G | BG | P | Y | Other | W | D | ||||||
| Europe | 2018 | Austria | M | 3 | 20 | 10 | 0 | 10 | 0 | 10 | 10 | 20 | 0 | 0 | 0 | 0 | 0 | 0 | 20 |
| Europe | 2018 | England | M | 3 | 8 | 4 | 0 | 4 | 0 | 6 | 2 | 8 | 0 | 0 | 0 | 0 | 0 | 0 | 8 |
| Europe | 2018 | France | M,P | 32 | 97 | 18 | 0 | 79 | 0 | 41 | 56 | 78 | 7 | 7 | 3 | 0 | 0 | 0 | 97 |
| Europe | 2018 | Netherlands | M | 3 | 18 | 6 | 0 | 12 | 0 | 9 | 9 | 9 | 0 | 0 | 0 | 0 | 0 | 18 | 0 |
| Europe | 2019 | Austria | H | 2 | 4 | 0 | 0 | 4 | 0 | 4 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 4 |
| Europe | 2019 | Bulgaria | H | 2 | 3 | 0 | 0 | 3 | 0 | 3 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| Europe | 2019 | Czech Rep | H | 2 | 32 | 0 | 0 | 32 | 0 | 32 | 0 | 32 | 0 | 0 | 0 | 0 | 0 | 32 | 0 |
| Europe | 2019 | England | H | 2 | 4 | 2 | 0 | 2 | 0 | 4 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 4 |
| Europe | 2019 | France | C, H, P, S | 26 | 170 | 0 | 0 | 170 | 0 | 164 | 6 | 89 | 59 | 13 | 9 | 0 | 0 | 88 | 82 |
| Europe | 2019 | Greece | H | 2 | 3 | 0 | 0 | 3 | 0 | 3 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| Europe | 2019 | Italy | H, S | 19 | 192 | 0 | 128 | 64 | 0 | 64 | 128 | 48 | 48 | 0 | 16 | 16 | 64 | 192 | 0 |
| Europe | 2019 | Portugal | H | 2 | 32 | 0 | 0 | 32 | 0 | 32 | 0 | 32 | 0 | 0 | 0 | 0 | 0 | 32 | 0 |
| Europe | 2019 | Spain | H | 1 | 2 | 0 | 0 | 2 | 0 | 2 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Europe | 2019 | Sweden | H | 1 | 16 | 0 | 0 | 16 | 0 | 16 | 0 | 16 | 0 | 0 | 0 | 0 | 0 | 16 | 0 |
| Europe | 2019 | Switzerland | H | 2 | 64 | 0 | 0 | 64 | 0 | 64 | 0 | 32 | 32 | 0 | 0 | 0 | 0 | 64 | 0 |
| Europe | 2020 | France | C, H, P, S | 48 | 166 | 2 | 0 | 64 | 100 | 160 | 6 | 77 | 35 | 17 | 17 | 17 | 0 | 18 | 148 |
| Europe | 2020 | Greece | H | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Europe | 2020 | Italy | H | 2 | 8 | 0 | 0 | 8 | 0 | 8 | 0 | 2 | 4 | 0 | 2 | 0 | 0 | 0 | 8 |
| Europe | 2020 | Portugal | H | 2 | 8 | 0 | 0 | 8 | 0 | 8 | 0 | 4 | 4 | 0 | 0 | 0 | 0 | 0 | 8 |
| Europe | 2020 | Spain | H | 6 | 22 | 0 | 0 | 22 | 0 | 22 | 0 | 22 | 0 | 0 | 0 | 0 | 0 | 0 | 22 |
| Europe | 2020 | Switzerland | H | 2 | 4 | 0 | 0 | 4 | 0 | 4 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 4 | 0 |
| Europe | 2021 | France | C, H, P, S | 58 | 165 | 2 | 0 | 58 | 105 | 162 | 3 | 98 | 42 | 0 | 14 | 11 | 0 | 30 | 135 |
| Europe | 2021 | Greece | H | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Europe | 2021 | Italy | H | 1 | 4 | 0 | 0 | 4 | 0 | 4 | 0 | 0 | 2 | 0 | 2 | 0 | 0 | 0 | 4 |
| Europe | 2021 | Portugal | H | 2 | 8 | 0 | 0 | 8 | 0 | 8 | 0 | 4 | 4 | 0 | 0 | 0 | 0 | 0 | 8 |
| Europe | 2021 | Slovenia | H | 4 | 18 | 0 | 0 | 18 | 0 | 18 | 0 | 18 | 0 | 0 | 0 | 0 | 0 | 0 | 18 |
| Europe | 2021 | Spain | H | 6 | 16 | 0 | 0 | 16 | 0 | 16 | 0 | 12 | 4 | 0 | 0 | 0 | 0 | 0 | 16 |
| Europe | 2021 | Switzerland | H | 9 | 19 | 8 | 0 | 11 | 0 | 19 | 0 | 8 | 11 | 0 | 0 | 0 | 0 | 11 | 8 |
| Europe | Total | 244 | 1105 | 52 | 128 | 720 | 205 | 885 | 220 | 631 | 252 | 37 | 63 | 44 | 64 | 505 | 600 | ||
| Asia | 2019 | China/Beijing | H | 1 | 3 | 0 | 0 | 3 | 0 | 3 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| Asia | 2019 | China/Hebei | H | 2 | 6 | 0 | 0 | 6 | 0 | 6 | 0 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 6 |
| Asia | 2019 | China/InnerMongolia | H | 1 | 3 | 0 | 0 | 3 | 0 | 3 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| Asia | 2019 | China/Liaoning | H | 1 | 3 | 0 | 0 | 3 | 0 | 3 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| Asia | 2019 | China/Yunnan | H | 1 | 4 | 0 | 0 | 4 | 0 | 4 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 4 |
| Asia | 2019 | China/Zhejiang | H | 1 | 3 | 0 | 0 | 3 | 0 | 3 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| Asia | 2020 | China/Hebei | H | 4 | 12 | 0 | 0 | 12 | 0 | 12 | 0 | 12 | 0 | 0 | 0 | 0 | 0 | 0 | 12 |
| Asia | 2020 | China/Hunan | H | 1 | 3 | 0 | 0 | 3 | 0 | 3 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| Asia | 2020 | China/Inner Mongolia | H | 2 | 6 | 0 | 0 | 6 | 0 | 6 | 0 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 6 |
| Asia | 2020 | China/Jiangxi | H | 2 | 6 | 0 | 0 | 6 | 0 | 6 | 0 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 6 |
| Asia | 2020 | China/Liaoning | H | 1 | 3 | 0 | 0 | 3 | 0 | 3 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| Asia | 2020 | China/Shandong | H | 1 | 3 | 0 | 0 | 3 | 0 | 3 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| Asia | 2020 | China/Yunnan | H | 1 | 4 | 0 | 0 | 4 | 0 | 4 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 4 |
| Asia | 2020 | China/Zhejiang | H | 1 | 3 | 0 | 0 | 3 | 0 | 3 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| Asia | 2021 | China/Beijing | H | 1 | 3 | 0 | 0 | 0 | 3 | 3 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| Asia | 2021 | China/Gansu | H | 1 | 3 | 0 | 0 | 3 | 0 | 3 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| Asia | 2021 | China/Hebei | H | 2 | 6 | 0 | 0 | 6 | 0 | 6 | 0 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 6 |
| Asia | 2021 | China/Hunan | H | 1 | 3 | 0 | 0 | 3 | 0 | 3 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| Asia | 2021 | China/Inner Mongolia | H | 2 | 6 | 0 | 0 | 3 | 3 | 6 | 0 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 6 |
| Asia | 2021 | China/Jiangxi | H | 2 | 6 | 0 | 0 | 6 | 0 | 6 | 0 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 6 |
| Asia | 2021 | China/Lioaning | H | 1 | 3 | 0 | 0 | 3 | 0 | 3 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| Asia | 2021 | China/Shandong | H | 1 | 3 | 0 | 0 | 3 | 0 | 3 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| Asia | 2021 | China/Yunnan | H | 3 | 5 | 0 | 0 | 0 | 5 | 5 | 0 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 5 |
| Asia | 2021 | China/Zhejiang | H | 1 | 3 | 0 | 0 | 3 | 0 | 3 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| Asia | 2021 | Russia/Siberia | H | 3 | 3 | 3 | 0 | 0 | 0 | 3 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| Asia | Total | 38 | 106 | 3 | 0 | 92 | 11 | 106 | 0 | 106 | 0 | 0 | 0 | 0 | 0 | 0 | 106 | ||
| North America | 2019 | USA/ Michigan | H | 9 | 18 | 18 | 0 | 0 | 0 | 18 | 0 | 18 | 0 | 0 | 0 | 0 | 0 | 18 | 0 |
| North America | 2019 | USA/ Ohio | H | 1 | 32 | 0 | 0 | 32 | 0 | 32 | 0 | 16 | 16 | 0 | 0 | 0 | 0 | 32 | 0 |
| North America | 2019 | Canada/ Nova Scotia | H | 1 | 32 | 0 | 0 | 32 | 0 | 32 | 0 | 16 | 16 | 0 | 0 | 0 | 0 | 32 | 0 |
| North America | Total | 11 | 82 | 18 | 0 | 64 | 0 | 82 | 0 | 50 | 32 | 0 | 0 | 0 | 0 | 82 | 0 | ||
| The Caribbean | 2020 | France/ Martinique | H | 2 | 3 | 3 | 0 | 0 | 0 | 3 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| The Caribbean | 2021 | France/ Martinique | H | 3 | 4 | 4 | 0 | 0 | 0 | 4 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 4 |
| The Caribbean | Total | 5 | 7 | 7 | 0 | 0 | 0 | 7 | 0 | 7 | 0 | 0 | 0 | 0 | 0 | 0 | 7 | ||
| Australia | 2020 | Australia | H | 2 | 4 | 0 | 0 | 4 | 0 | 4 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 4 |
| Australia | 2021 | Australia | H | 2 | 4 | 0 | 0 | 4 | 0 | 4 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 4 |
| Australia | Total | 4 | 8 | 0 | 0 | 8 | 0 | 8 | 0 | 8 | 0 | 0 | 0 | 0 | 0 | 0 | 8 | ||
| 0 | |||||||||||||||||||
| Grand total | 302 | 1308 | 80 | 128 | 884 | 216 | 1088 | 220 | 802 | 284 | 37 | 63 | 44 | 64 | 587 | 721 | |||
In 2018, trials were limited to four European countries (Austria, England, France, the Netherlands), including 41 sites with 143 traps. The 2019 trials were much more extensive and involved 12 European countries (the four from 2018, supplemented by Bulgaria, the Czech Republic, Greece, Italy, Portugal, Spain, Sweden and Switzerland), five provinces of China (Beijing, Hebei, Liaoning, Yunnan and Zhejiang), two States of the USA (Michigan and Ohio) and one site in Canada (Nova Scotia), resulting in a total of 79 sites and 626 traps. These 2019 trials included a large trapping programme targeting semi-urban forests located close to ports-of-entry in Europe, USA and Canada where 16 (Czech Republic, Portugal, Sweden) or 32 traps (France, Italy, Nova Scotia, Ohio, Switzerland) were deployed at each target site. The 2020 trials were substantially impacted by the COVID-19 pandemic, but were carried out for at least a part of the spring–summer season in six European countries (France, Greece, Italy, Portugal, Spain and Switzerland), eight provinces of China (those of 2019, except Beijing, to which were added Hunan, Inner Mongolia, Jiangxi and Shandong) and extended to Australia (New South Wales) and the Caribbean (Martinique), resulting in a total of 78 sites and 256 traps. The 2021 trials were deployed in the same countries as in 2020, supplemented by an additional European country (Slovenia), Russia (Siberia) and an additional province of China (Gansu), resulting in a total of 104 sites and 283 traps.
Trapping protocol and 8-pheromone blend
Trials at all sites used either multifunnel or cross-vane panel traps supplied by different companies depending on the country (Econex, Spain; ChemTica Internacional, S.A., Heredia, Costa Rica; Alpha Scents Inc., West Linn, Oregon, USA). Cross-vane traps used in Italy (Colli Euganei area) in 2019 were hand-made (see
All lures were prepared by INRAE before being shipped to all study participants. These lures consisted of a blend designed by
Composition of the 8-pheromone and 10-pheromone blends and targeted sex and cerambycid tribes.
| Blend | Compound | Amount/lure (mg/ml) | Target Sex | Target subfamily | Target tribe/genus | References | |||
|---|---|---|---|---|---|---|---|---|---|
| Cerambycinae | Lamiinae | Aseminae | Prioninae | ||||||
| 8-pheromones | Racemic 3-hydroxyhexan-2-one (C6-ketol) | 50 | M/F | X | Callidiini |
|
|||
| X | Clytini |
|
|||||||
| X | Hesperophanini | unpub data JGM | |||||||
| X | Hylotrupini |
|
|||||||
| 8-pheromones | Racemic 2-methylbutan-1-ol | 50 | M/F | X | Callidiini |
|
|||
| 8-pheromones | 2R*,3S*-2,3-hexanediol | 50 | M/F | X | Clytini |
|
|||
| 8-pheromones | Racemic fuscumol + fuscumol acetate | 50+ 50 | M/F | X | Obriini |
|
|||
| X | Acanthocinini |
|
|||||||
| X | Acanthoderini |
|
|||||||
| X | Asemini |
|
|||||||
| 8-pheromones | Monochamol | 50 | M/F | X | Monochamini |
|
|||
| X | Lamiini |
|
|||||||
| 8-pheromones | Geranylacetone | 25 | M/F | X | Acanthocinini |
|
|||
| X | Asemini |
|
|||||||
| 8-pheromones | Prionic acid (4 stereoisomers) | 05 | M | X | Prionini |
|
|||
| 10-pheromones | Racemic trichoferone | 25 | M/F | X | Trichoferus |
|
|||
| 10-pheromones | (E)-2-cis-6,7-epoxynonenal | 50 | M/F | X | Aromia |
|
|||
All primary compounds were obtained from ChemTica Internacional, except prionic acid, which was purchased from Alpha Scents Inc. Commercial high release rate ethanol (100 ml dose, 96% purity, release rate 2 g/day at 20 °C; Econex, Spain) and α-pinene lures (25 ml dose, 98% purity, release rate 0.3 g/day at 20 °C; Econex, Spain) were added to traps in most trials (1076 of the 1308 traps; Table
In most cases, the trapped insects were killed using a section of mesh impregnated with α-cypermethrin insecticide (Storanet, BASF Pflanzenschutz Deutschland, Germany) placed into the trap basins, whose bottoms had been replaced with a wire mesh to allow drainage and to keep specimens dry. However, in the targeted 2019 experiment in forests near ports-of-entry and in the Colli Euganei area (Italy), “wet” trap basins were used, containing water-diluted propylene glycol (50%) to act as a surfactant and preservative. In the trials conducted in Ohio and Michigan, trap collection cups were filled with ~ 200–400 ml of undiluted propylene glycol.
Trapped cerambycids were identified to species by local specialists or sent to INRAE for identification. However, specimens trapped in Australia could not be sent due to restrictions by the customs agency and so most could only be identified to the genus level. Nomenclature used in this article follows the reference checklist of the world database Titan (
Preliminary tests of a 10-pheromone blend
In 2020 and 2021, two additional pheromones, trichoferone (the pheromone of T. campestris) and (E)-2-cis-6,7-epoxynonenal (the pheromone of A. bungii), were added to the 8-pheromone lures used in France and China, to test for a possible increase in monitoring effectiveness with a 10-pheromone blend (Table
Results
A total of 78,321 longhorned beetles were trapped, representing 376 species, including 373 Cerambycidae, two Vesperidae and one Disteniidae species (Table
Names of trapped species, origin and specimen numbers captured per continent. Species in bold were trapped in non-native continents.
| Subfamily | Tribe | Species | Origin | Europe | Asia | North America | The Caribbean | Australia | Total |
|---|---|---|---|---|---|---|---|---|---|
| Cerambycinae | Anaglyptini | Anaglyptus gibbosus (Fabricius, 1787) | Europe | 105 | 0 | 0 | 0 | 0 | 105 |
| Cerambycinae | Anaglyptini | Anaglyptus mysticus (Linnaeus, 1758) | Europe | 116 | 0 | 0 | 0 | 0 | 116 |
| Cerambycinae | Anaglyptini | Cyrtophorus verrucosus (Olivier, 1800) | North America | 0 | 0 | 197 | 0 | 0 | 197 |
| Cerambycinae | Anaglyptini | Microclytus compressicollis (Laporte de Castelnau & Gory, 1841) | North America | 0 | 0 | 2 | 0 | 0 | 2 |
| Cerambycinae | Bothriospilini | Chlorida festiva (Linnaeus, 1758) | Caribbean | 0 | 0 | 0 | 0 | 2 | 2 |
| Cerambycinae | Callichromatini | Aromia bungii Faldermann, 1835 | Asia | 0 | 25 | 0 | 0 | 0 | 25 |
| Cerambycinae | Callichromatini | Aromia moschata (Linnaeus, 1758) | Europe | 30 | 0 | 0 | 0 | 0 | 30 |
| Cerambycinae | Callichromatini | Aromia moschata orientalis Plavilstshikov, 1933 | Asia | 0 | 3 | 0 | 0 | 0 | 3 |
| Cerambycinae | Callidiini | Callidium aeneum (Degeer, 1775) | Holarctic | 120 | 79 | 0 | 0 | 0 | 199 |
| Cerambycinae | Callidiini | Callidium violaceum (Linnaeus, 1758) | Europe | 3 | 0 | 0 | 0 | 0 | 3 |
| Cerambycinae | Callidiini | Lioderina linearis (Hampe, 1870) | Europe | 3 | 0 | 0 | 0 | 0 | 3 |
| Cerambycinae | Callidiini | Phymatodes aereus (Newman, 1838) | North America | 0 | 0 | 14 | 0 | 0 | 14 |
| Cerambycinae | Callidiini | Phymatodes alni (Linnaeus, 1767) | Europe | 2295 | 0 | 0 | 0 | 0 | 2295 |
| Cerambycinae | Callidiini | Phymatodes amoenus (Say, 1824) | North America | 0 | 0 | 3100 | 0 | 0 | 3100 |
| Cerambycinae | Callidiini | Phymatodes dimidiatus (Kirby, 1837) | North America | 0 | 0 | 55 | 0 | 0 | 55 |
| Cerambycinae | Callidiini | Phymatodes fasciatus (Villers, 1789) | Europe | 6 | 0 | 0 | 0 | 0 | 6 |
| Cerambycinae | Callidiini | Phymatodes glabratus (Charpentier, 1825) | Europe | 3 | 0 | 0 | 0 | 0 | 3 |
| Cerambycinae | Callidiini | Phymatodes lividus (Rossi, 1794) | Europe | 7 | 0 | 0 | 0 | 0 | 7 |
| Cerambycinae | Callidiini | Phymatodes pusillus (Fabricius, 1787) | Europe | 37 | 0 | 0 | 0 | 0 | 37 |
| Cerambycinae | Callidiini | Phymatodes rufipes (Fabricius, 1776) | Europe | 3 | 0 | 0 | 0 | 0 | 3 |
| Cerambycinae | Callidiini | Phymatodes testaceus (Linnaeus, 1758) | Europe | 15085 | 0 | 41 | 0 | 0 | 15126 |
| Cerambycinae | Callidiini | Phymatodes varius (Fabricius, 1776) | North America | 0 | 0 | 29 | 0 | 0 | 29 |
| Cerambycinae | Callidiini | Physocnemum brevilineum (Say, 1824) | North America | 0 | 0 | 4 | 0 | 0 | 4 |
| Cerambycinae | Callidiini | Pyrrhidium sanguineum (Linnaeus, 1758) | Europe | 4388 | 0 | 0 | 0 | 0 | 4388 |
| Cerambycinae | Callidiini | Ropalopus clavipes (Fabricius, 1775) | Europe | 69 | 0 | 0 | 0 | 0 | 69 |
| Cerambycinae | Callidiini | Ropalopus femoratus (Linnaeus, 1758) | Europe | 35 | 0 | 0 | 0 | 0 | 35 |
| Cerambycinae | Callidiini | Ropalopus macropus (Germar, 1823) | Europe | 21 | 0 | 0 | 0 | 0 | 21 |
| Cerambycinae | Callidiini | Ropalopus varini (Bedel, 1870) | Europe | 1 | 0 | 0 | 0 | 0 | 1 |
| Cerambycinae | Callidiopini | Bethelium sp. | Australasia | 0 | 0 | 0 | 5 | 0 | 5 |
| Cerambycinae | Callidiopini | Curtomerus flavus (Fabricius, 1775) | Caribbean | 0 | 0 | 0 | 0 | 7 | 7 |
| Cerambycinae | Callidiopini | Stenodryas clavigera Bates, 1873 | Asia | 0 | 1 | 0 | 0 | 0 | 1 |
| Cerambycinae | Cerambycini | Cerambyx cerdo Linnaeus, 1758 | Europe | 20 | 0 | 0 | 0 | 0 | 20 |
| Cerambycinae | Cerambycini | Cerambyx miles Bonelli, 1812 | Europe | 3 | 0 | 0 | 0 | 0 | 3 |
| Cerambycinae | Cerambycini | Cerambyx scopolii Fueßlins, 1775 | Europe | 141 | 0 | 0 | 0 | 0 | 141 |
| Cerambycinae | Cerambycini | Cerambyx welensii (Küster, 1845) | Europe | 22 | 0 | 0 | 0 | 0 | 22 |
| Cerambycinae | Cerambycini | Nadezhdiella cantori (Hope, 1842) | Asia | 0 | 1 | 0 | 0 | 0 | 1 |
| Cerambycinae | Clytini | Chlorophorus figuratus (Scopoli, 1763) | Europe | 42 | 0 | 0 | 0 | 0 | 42 |
| Cerambycinae | Clytini | Chlorophorus glabromaculatus (Goeze, 1777) | Europe | 1391 | 0 | 0 | 0 | 0 | 1391 |
| Cerambycinae | Clytini | Chlorophorus glaucus (Fabricius, 1781) | Europe | 36 | 0 | 0 | 0 | 0 | 36 |
| Cerambycinae | Clytini | Chlorophorus herbstii (Brahm, 1790) | Europe | 6 | 0 | 0 | 0 | 0 | 6 |
| Cerambycinae | Clytini | Chlorophorus miwai Gressitt, 1936 | Asia | 0 | 9 | 0 | 0 | 0 | 9 |
| Cerambycinae | Clytini | Chlorophorus motschulskyi (Ganglbauer, 1887) | Asia | 0 | 7 | 0 | 0 | 0 | 7 |
| Cerambycinae | Clytini | Chlorophorus ruficornis (Olivier, 1790) | Europe | 41 | 0 | 0 | 0 | 0 | 41 |
| Cerambycinae | Clytini | Chlorophorus sartor (Müller, 1766) | Europe | 482 | 0 | 0 | 0 | 0 | 482 |
| Cerambycinae | Clytini | Chlorophorus signaticollis (Laporte de Castelnau & Gory, 1836) | Asia | 0 | 1 | 0 | 0 | 0 | 1 |
| Cerambycinae | Clytini | Chlorophorus tredecimmaculatus (Chevrolat, 1863) | Asia | 0 | 2 | 0 | 0 | 0 | 2 |
| Cerambycinae | Clytini | Chlorophorus trifasciatus (Fabricius, 1781) | Europe | 33 | 0 | 0 | 0 | 0 | 33 |
| Cerambycinae | Clytini | Chlorophorus varius (Müller, 1766) | Europe | 36 | 0 | 0 | 0 | 0 | 36 |
| Cerambycinae | Clytini | Clytoleptus albofasciatus (Laporte de Castelnau & Gory, 1841) | North America | 0 | 0 | 6 | 0 | 0 | 6 |
| Cerambycinae | Clytini | Clytus arietis (Linnaeus, 1758) | Europe | 52 | 0 | 0 | 0 | 0 | 52 |
| Cerambycinae | Clytini | Clytus lama Mulsant, 1850 | Europe | 123 | 0 | 0 | 0 | 0 | 123 |
| Cerambycinae | Clytini | Clytus rhamni Germar, 1817 | Europe | 85 | 0 | 0 | 0 | 0 | 85 |
| Cerambycinae | Clytini | Clytus ruricola (Olivier, 1800) | North America | 0 | 0 | 25 | 0 | 0 | 25 |
| Cerambycinae | Clytini | Clytus tropicus (Panzer, 1795) | Europe | 73 | 0 | 0 | 0 | 0 | 73 |
| Cerambycinae | Clytini | Cyrtoclytus capra (Germar, 1823) | Asia | 0 | 24 | 0 | 0 | 0 | 24 |
| Cerambycinae | Clytini | Cyrtoclytus caproides (Bates, 1873) | Asia | 0 | 5 | 0 | 0 | 0 | 5 |
| Cerambycinae | Clytini | Demonax diversefasciatus Pic, 1920 | Asia | 0 | 1 | 0 | 0 | 0 | 1 |
| Cerambycinae | Clytini | Demonax nansenensis Pic 1903 | Asia | 0 | 1 | 0 | 0 | 0 | 1 |
| Cerambycinae | Clytini | Demonax sp. 1 | Asia | 0 | 1 | 0 | 0 | 0 | 1 |
| Cerambycinae | Clytini | Demonax sp. 2 | Asia | 0 | 1 | 0 | 0 | 0 | 1 |
| Cerambycinae | Clytini | Glycobius speciosus (Say, 1824) | North America | 0 | 0 | 1 | 0 | 0 | 1 |
| Cerambycinae | Clytini | Isotomus speciosus (Schneider, 1787) | Europe | 1 | 0 | 0 | 0 | 0 | 1 |
| Cerambycinae | Clytini | Megacyllene caryae (Gahan, 1908) | North America | 0 | 0 | 22 | 0 | 0 | 22 |
| Cerambycinae | Clytini | Neoclytus acuminatus acuminatus (Fabricius, 1775) | North America | 37 | 0 | 28 | 0 | 0 | 65 |
| Cerambycinae | Clytini | Neoclytus caprea (Say, 1824) | North America | 0 | 0 | 3 | 0 | 0 | 3 |
| Cerambycinae | Clytini | Neoclytus leucozonus (Laporte de Castelnau & Gory, 1841) | North America | 0 | 0 | 15 | 0 | 0 | 15 |
| Cerambycinae | Clytini | Neoclytus mucronatus mucronatus (Fabricius, 1775) | North America | 0 | 0 | 323 | 0 | 0 | 323 |
| Cerambycinae | Clytini | Neoclytus muricatulus (Kirby, 1837) | North America | 0 | 0 | 1 | 0 | 0 | 1 |
| Cerambycinae | Clytini | Neoclytus scutellaris (Olivier, 1790) | North America | 0 | 0 | 2 | 0 | 0 | 2 |
| Cerambycinae | Clytini | Perissus paulonotatus (Pic, 1902) | Asia | 0 | 21 | 0 | 0 | 0 | 21 |
| Cerambycinae | Clytini | Plagionotus arcuatus (Linnaeus, 1758) | Europe | 95 | 0 | 0 | 0 | 0 | 95 |
| Cerambycinae | Clytini | Plagionotus christophi (Kraatz, 1879) | Asia | 0 | 1 | 0 | 0 | 0 | 1 |
| Cerambycinae | Clytini | Plagionotus detritus (Linnaeus, 1758) | Europe | 299 | 0 | 0 | 0 | 0 | 299 |
| Cerambycinae | Clytini | Pseudosphegesthes cinerea (Laporte de Castelnau & Gory, 1841) | Europe | 27 | 0 | 0 | 0 | 0 | 27 |
| Cerambycinae | Clytini | Raphuma anongi Gressitt & Rondon, 1970 | Asia | 0 | 96 | 0 | 0 | 0 | 96 |
| Cerambycinae | Clytini | Raphuma gracilipes (Faldermann, 1835) | Asia | 0 | 24 | 0 | 0 | 0 | 24 |
| Cerambycinae | Clytini | Raphuma laosica Gressitt & Rondon, 1970 | Asia | 0 | 22 | 0 | 0 | 0 | 22 |
| Cerambycinae | Clytini | Raphuma sp. | Asia | 0 | 1 | 0 | 0 | 0 | 1 |
| Cerambycinae | Clytini | Rhabdoclytus acutivittis (Kraatz, 1879) | Asia | 0 | 1 | 0 | 0 | 0 | 1 |
| Cerambycinae | Clytini | Sarosesthes fulminans (Fabricius, 1775) | North America | 0 | 0 | 39 | 0 | 0 | 39 |
| Cerambycinae | Clytini | Xylotrechus antilope (Schönherr, 1817) | Europe | 1303 | 0 | 0 | 0 | 0 | 1303 |
| Cerambycinae | Clytini | Xylotrechus antilope var sekerai Podaný, 1970 | Europe | 16 | 0 | 0 | 0 | 0 | 16 |
| Cerambycinae | Clytini | Xylotrechus arvicola (Olivier, 1800) | Europe | 379 | 0 | 0 | 0 | 0 | 379 |
| Cerambycinae | Clytini | Xylotrechus atronotatus Pic, 1917 | Asia | 0 | 1 | 0 | 0 | 0 | 1 |
| Cerambycinae | Clytini | Xylotrechus buqueti (Laporte de Castelnau & Gory, 1841) | Asia | 0 | 38 | 0 | 0 | 0 | 38 |
| Cerambycinae | Clytini | Xylotrechus chinensis (Chevrolat, 1852) | Asia | 41 | 3 | 0 | 0 | 0 | 44 |
| Cerambycinae | Clytini | Xylotrechus clarinus Bates, 1884 | Asia | 0 | 1 | 0 | 0 | 0 | 1 |
| Cerambycinae | Clytini | Xylotrechus colonus (Fabricius, 1775) | North America | 0 | 0 | 484 | 0 | 0 | 484 |
| Cerambycinae | Clytini | Xylotrechus gratus Viktora, 2020 | Asia | 0 | 1 | 0 | 0 | 0 | 1 |
| Cerambycinae | Clytini | Xylotrechus integer (Haldeman, 1847) | North America | 0 | 0 | 1 | 0 | 0 | 1 |
| Cerambycinae | Clytini | Xylotrechus latefasciatus ochroceps Gressitt, 1951 | Asia | 0 | 1 | 0 | 0 | 0 | 1 |
| Cerambycinae | Clytini | Xylotrechus magnicollis (Fairmaire, 1888) | Asia | 0 | 1 | 0 | 0 | 0 | 1 |
| Cerambycinae | Clytini | Xylotrechus pantherinus (Savenius, 1825) | Europe | 1 | 0 | 0 | 0 | 0 | 1 |
| Cerambycinae | Clytini | Xylotrechus pekingensis Pic, 1939 | Asia | 0 | 1 | 0 | 0 | 0 | 1 |
| Cerambycinae | Clytini | Xylotrechus rufilius Bates, 1884 | Asia | 0 | 27 | 0 | 0 | 0 | 27 |
| Cerambycinae | Clytini | Xylotrechus rusticus (Linnaeus, 1758) | Europe/Asia | 161 | 1 | 0 | 0 | 0 | 162 |
| Cerambycinae | Clytini | Xylotrechus sagittatus (Germar, 1821) | North America | 0 | 0 | 34 | 0 | 0 | 34 |
| Cerambycinae | Clytini | Xylotrechus stebbingi Gahan, 1906 | Asia | 6089 | 0 | 0 | 0 | 0 | 6054 |
| Cerambycinae | Clytini | Xylotrechus undulatus (Say, 1824) | North America | 0 | 0 | 26 | 0 | 0 | 26 |
| Cerambycinae | Deilini | Deilus fugax (Olivier, 1790) | Europe | 87 | 0 | 0 | 0 | 0 | 87 |
| Cerambycinae | Dryobiini | Dryobius sexnotatus Linsley, 1957 | North America | 0 | 0 | 2 | 0 | 0 | 2 |
| Cerambycinae | Eburiini | Eburia dejeani Gahan, 1895 | Caribbean | 0 | 0 | 0 | 0 | 2 | 2 |
| Cerambycinae | Eburiini | Eburia octomaculata Chevrolat, 1862 | Caribbean | 0 | 0 | 0 | 0 | 1 | 1 |
| Cerambycinae | Eburiini | Eburia quadrigeminata (Say, 1827) | North America | 0 | 0 | 3 | 0 | 0 | 3 |
| Cerambycinae | Elaphidiini | Anelaphus pumilus (Newman, 1840) | North America | 0 | 0 | 531 | 0 | 0 | 531 |
| Cerambycinae | Elaphidiini | Anelaphus villosus (Fabricius, 1793) | North America | 0 | 0 | 8 | 0 | 0 | 8 |
| Cerambycinae | Elaphidiini | Elaphidion mucronatum (Say, 1824) | North America | 0 | 0 | 110 | 0 | 0 | 110 |
| Cerambycinae | Elaphidiini | Parelaphidion aspersum (Haldeman, 1847) | North America | 0 | 0 | 3 | 0 | 0 | 3 |
| Cerambycinae | Elaphidiini | Parelaphidion incertum (Newman, 1840) | North America | 0 | 0 | 4 | 0 | 0 | 4 |
| Cerambycinae | Graciliini | Axinopalpis gracilis (Krynicki, 1832) | Europe | 8 | 0 | 0 | 0 | 0 | 8 |
| Cerambycinae | Graciliini | Gracilia minuta (Fabricius, 1781) | Europe | 12 | 0 | 0 | 0 | 0 | 12 |
| Cerambycinae | Graciliini | Penichroa fasciata (Stephens, 1831) | Europe | 41 | 0 | 0 | 0 | 0 | 41 |
| Cerambycinae | Hesperophanini | Gnatholea eburifera Thomson, 1861 | Asia | 0 | 10 | 0 | 0 | 0 | 10 |
| Cerambycinae | Hesperophanini | Hesperophanes sericeus (Fabricius, 1787) | Europe | 8 | 0 | 0 | 0 | 0 | 8 |
| Cerambycinae | Hesperophanini | Stromatium auratum (Böber, 1793) | Europe | 7 | 0 | 0 | 0 | 0 | 7 |
| Cerambycinae | Hesperophanini | Trichoferus campestris (Faldermann, 1835) | Asia | 45 | 12 | 0 | 0 | 0 | 57 |
| Cerambycinae | Hesperophanini | Trichoferus fasciculatus (Faldermann, 1837) | Europe | 135 | 0 | 0 | 0 | 0 | 135 |
| Cerambycinae | Hesperophanini | Trichoferus guerryi (Pic, 1915) | Asia | 0 | 1 | 0 | 0 | 0 | 1 |
| Cerambycinae | Hesperophanini | Trichoferus holosericeus (Rossi, 1790) | Europe | 187 | 0 | 0 | 0 | 0 | 187 |
| Cerambycinae | Hesperophanini | Trichoferus pallidus (Olivier, 1790) | Europe | 145 | 0 | 0 | 0 | 0 | 145 |
| Cerambycinae | Hylotrupini | Hylotrupes bajulus (Linnaeus, 1758) | Europe | 79 | 0 | 0 | 0 | 0 | 79 |
| Cerambycinae | Molorchini | Dolocerus reichii Mulsant, 1862 | Europe | 1 | 0 | 0 | 0 | 0 | 1 |
| Cerambycinae | Molorchini | Molorchus bimaculatus Say, 1824 | North America | 0 | 0 | 122 | 0 | 0 | 122 |
| Cerambycinae | Molorchini | Molorchus minor (Linnaeus, 1758) | Europe | 15 | 0 | 0 | 0 | 0 | 15 |
| Cerambycinae | Molorchini | Molorchus umbellatarum (Schreber, 1759) | Europe | 55 | 0 | 0 | 0 | 0 | 55 |
| Cerambycinae | Neoibidionini | Neocompsa cylindricollis (Fabricius, 1798) | Caribbean | 0 | 0 | 0 | 0 | 1 | 1 |
| Cerambycinae | Obriini | Obrium brunneum (Fabricius, 1793) | Europe | 3 | 0 | 0 | 0 | 0 | 3 |
| Cerambycinae | Obriini | Obrium cantharinum (Linnaeus, 1767) | Europe | 44 | 0 | 0 | 0 | 0 | 44 |
| Cerambycinae | Obriini | Obrium maculatum (Olivier, 1800) | North America | 0 | 0 | 1 | 0 | 0 | 1 |
| Cerambycinae | Phoracanthini | Cordylomera spinicornis (Fabricius, 1775) | Africa | 4 | 0 | 0 | 0 | 0 | 4 |
| Cerambycinae | Phoracanthini | Phoracantha recurva Newman, 1840 | Australasia | 8 | 0 | 0 | 0 | 0 | 8 |
| Cerambycinae | Phoracanthini | Phoracantha semipunctata (Fabricius, 1775) | Australasia | 11 | 0 | 0 | 0 | 0 | 11 |
| Cerambycinae | Phoracanthini | Thoris sp. | Australasia | 0 | 0 | 0 | 2 | 0 | 2 |
| Cerambycinae | Psebiini | Nathrius brevipennis (Mulsant, 1839) | Europe | 649 | 0 | 0 | 0 | 0 | 649 |
| Cerambycinae | Pytheini | Certallum ebulinum (Linnaeus, 1767) | Europe | 1 | 0 | 0 | 0 | 0 | 1 |
| Cerambycinae | Rhopalophorini | Rhopalophora longipes (Say, 1824) | North America | 0 | 0 | 1 | 0 | 0 | 1 |
| Cerambycinae | Stenhomalini | Stenhomalus fenestratus White,1855 | Asia | 0 | 3 | 0 | 0 | 0 | 3 |
| Cerambycinae | Stenoderini | Syllitus sp. | Australasia | 0 | 0 | 0 | 2 | 0 | 2 |
| Cerambycinae | Stenopterini | Callimoxys sanguinicollis (Olivier, 1800) | North America | 0 | 0 | 1 | 0 | 0 | 1 |
| Cerambycinae | Stenopterini | Callimus abdominalis (Olivier, 1800) | Europe | 11 | 0 | 0 | 0 | 0 | 11 |
| Cerambycinae | Stenopterini | Callimus angulatus (Schrank, 1789) | Europe | 1 | 0 | 0 | 0 | 0 | 1 |
| Cerambycinae | Stenopterini | Stenopterus ater (Linnaeus, 1767) | Europe | 20 | 0 | 0 | 0 | 0 | 20 |
| Cerambycinae | Stenopterini | Stenopterus rufus (Linnaeus, 1767) | Europe | 83 | 0 | 0 | 0 | 0 | 83 |
| Cerambycinae | Tillomorphini | Bonfilsia pejoti Chalumeau & Touroult, 2004 | Caribbean | 0 | 0 | 0 | 0 | 1 | 1 |
| Cerambycinae | Tillomorphini | Euderces picipes (Fabricius, 1787) | North America | 0 | 0 | 9 | 0 | 0 | 9 |
| Cerambycinae | Tillomorphini | Euderces pini (Olivier, 1800) | North America | 0 | 0 | 93 | 0 | 0 | 93 |
| Cerambycinae | Tillomorphini | Gourbeyrella madininae Chalumeau & Touroult, 2004 | Caribbean | 0 | 0 | 0 | 0 | 3 | 3 |
| Cerambycinae | Trachyderini | Anoplistes halodendri (Pallas, 1773) | Asia | 0 | 2 | 0 | 0 | 0 | 2 |
| Cerambycinae | Trachyderini | Dicelosternus corallinus Gahan, 1900 | Asia | 0 | 3 | 0 | 0 | 0 | 3 |
| Cerambycinae | Trachyderini | Purpuricenus budensis (Götz, 1783) | Europe | 18 | 0 | 0 | 0 | 0 | 18 |
| Cerambycinae | Trachyderini | Purpuricenus globulicollis Dejean, 1839 | Europe | 2 | 0 | 0 | 0 | 0 | 2 |
| Cerambycinae | Trachyderini | Purpuricenus kaehleri (Linnaeus, 1758) | Europe | 261 | 0 | 0 | 0 | 0 | 261 |
| Cerambycinae | Trachyderini | Purpuricenus lituratus Ganglbauer, 1887 | Asia | 0 | 1 | 0 | 0 | 0 | 1 |
| Cerambycinae | Trachyderini | Purpuricenus temminckii (Guérin-Méneville, 1844) | Asia | 0 | 10 | 0 | 0 | 0 | 10 |
| Cerambycinae | Trachyderini | Amarysius altajensis (Laxmann, 1770) | Asia | 0 | 20 | 0 | 0 | 0 | 20 |
| Lamiinae | Acanthocinini | Acanthocinus aedilis (Linnaeus, 1758) | Europe/Asia | 6 | 24 | 0 | 0 | 0 | 30 |
| Lamiinae | Acanthocinini | Acanthocinus griseus (Fabricius, 1793) | Europe/Asia | 114 | 106 | 0 | 0 | 0 | 220 |
| Lamiinae | Acanthocinini | Acanthocinus pusillus (Kirby, 1837) | North America | 0 | 0 | 21 | 0 | 0 | 21 |
| Lamiinae | Acanthocinini | Amniscus similis (Gahan, 1895) | Caribbean | 0 | 0 | 0 | 0 | 5 | 5 |
| Lamiinae | Acanthocinini | Astyleiopus variegatus (Haldeman, 1847) | North America | 0 | 0 | 11 | 0 | 0 | 11 |
| Lamiinae | Acanthocinini | Astylidius parvus (LeConte, 1873) | North America | 0 | 0 | 17 | 0 | 0 | 17 |
| Lamiinae | Acanthocinini | Astylopsis macula (Say, 1827) | North America | 0 | 0 | 47 | 0 | 0 | 47 |
| Lamiinae | Acanthocinini | Astylopsis sexguttata (Say, 1827) | North America | 0 | 0 | 19 | 0 | 0 | 19 |
| Lamiinae | Acanthocinini | Astylopsis sp. | North America | 0 | 0 | 2 | 0 | 0 | 2 |
| Lamiinae | Acanthocinini | Graphisurus despectus (LeConte, 1850) | North America | 0 | 0 | 3 | 0 | 0 | 3 |
| Lamiinae | Acanthocinini | Graphisurus fasciatus (Degeer, 1775) | North America | 0 | 0 | 86 | 0 | 0 | 86 |
| Lamiinae | Acanthocinini | Graphisurus triangulifer (Haldeman, 1847) | North America | 0 | 0 | 3 | 0 | 0 | 3 |
| Lamiinae | Acanthocinini | Hyperplatys maculatus Haldeman, 1847 | North America | 0 | 0 | 4 | 0 | 0 | 4 |
| Lamiinae | Acanthocinini | Lagocheirus araneiformis insulorum Dillon, 1957 | Caribbean | 0 | 0 | 0 | 0 | 4 | 4 |
| Lamiinae | Acanthocinini | Leiopus fallaciosus Holzschuh, 1993 | Asia | 0 | 5 | 0 | 0 | 0 | 5 |
| Lamiinae | Acanthocinini | Leiopus femoratus Fairmaire, 1859 | Europe | 3461 | 0 | 0 | 0 | 0 | 3461 |
| Lamiinae | Acanthocinini | Leiopus linnei Wallin, Nýlander & Kvamme, 2009 | Europe | 548 | 0 | 0 | 0 | 0 | 548 |
| Lamiinae | Acanthocinini | Leiopus nebulosus (Linneus, 1758) | Europe | 1473 | 0 | 0 | 0 | 0 | 1473 |
| Lamiinae | Acanthocinini | Leptostylus transversus (Gyllenhal, 1817) | North America | 0 | 0 | 101 | 0 | 0 | 101 |
| Lamiinae | Acanthocinini | Lepturges angulatus (LeConte, 1852) | North America | 0 | 0 | 20 | 0 | 0 | 20 |
| Lamiinae | Acanthocinini | Lepturges confluens (Haldeman, 1847) | North America | 0 | 0 | 26 | 0 | 0 | 26 |
| Lamiinae | Acanthocinini | Lepturges sp. | North America | 0 | 0 | 2 | 0 | 0 | 2 |
| Lamiinae | Acanthocinini | Sternidius alpha (Say, 1827) | North America | 0 | 0 | 3 | 0 | 0 | 3 |
| Lamiinae | Acanthocinini | Sternidius punctatus (Haldeman, 1847) | North America | 0 | 0 | 1 | 0 | 0 | 1 |
| Lamiinae | Acanthocinini | Sternidius rusticus (LeConte, 1852) | North America | 0 | 0 | 19 | 0 | 0 | 19 |
| Lamiinae | Acanthocinini | Styloleptus posticalis (Gahan, 1895) | Caribbean | 0 | 0 | 0 | 0 | 1 | 1 |
| Lamiinae | Acanthocinini | Trypanidius spilmani Villiers, 1980 | Caribbean | 0 | 0 | 0 | 0 | 1 | 1 |
| Lamiinae | Acanthocinini | Urgleptes cobbeni Gilmour, 1963 | Caribbean | 0 | 0 | 0 | 0 | 1 | 1 |
| Lamiinae | Acanthocinini | Urgleptes querci (Fitch, 1859) | North America | 0 | 0 | 2 | 0 | 0 | 2 |
| Lamiinae | Acanthocinini | Urgleptes signatus (LeConte, 1852) | North America | 0 | 0 | 1 | 0 | 0 | 1 |
| Lamiinae | Acanthoderini | Aegomorphus clavipes (Schrank von Paula, 1781) | Europe | 1412 | 0 | 0 | 0 | 0 | 1412 |
| Lamiinae | Acanthoderini | Aegomorphus francottei Sama, 1994 | Europe | 181 | 0 | 0 | 0 | 0 | 181 |
| Lamiinae | Acanthoderini | Aegomorphus krueperi (Kraatz, 1859) | Europe | 7 | 0 | 0 | 0 | 0 | 7 |
| Lamiinae | Acanthoderini | Aegomorphus modestus (Blais, 1817) | North America | 0 | 0 | 58 | 0 | 0 | 58 |
| Lamiinae | Acanthoderini | Aegomorphus quadrigibbus (Say, 1831) | North America | 0 | 0 | 3 | 0 | 0 | 3 |
| Lamiinae | Acanthoderini | Oplosia cinerea (Mulsant, 1839) | Europe | 63 | 0 | 0 | 0 | 0 | 63 |
| Lamiinae | Acanthoderini | Oplosia nubila (LeConte, 1862) | North America | 0 | 0 | 4 | 0 | 0 | 4 |
| Lamiinae | Agapanthiini | Agapanthia cardui (Linnaeus, 1767) | Europe | 1 | 0 | 0 | 0 | 0 | 1 |
| Lamiinae | Agapanthiini | Agapanthia villosoviridescens (Degeer, 1775) | Europe | 1 | 0 | 0 | 0 | 0 | 1 |
| Lamiinae | Apomecynini | Apomecyna saltator (Fabricius, 1787) | Asia | 0 | 1 | 0 | 0 | 0 | 1 |
| Lamiinae | Ceroplesini | Moechotypa diphysis (Pascoe, 1871) | Asia | 0 | 2 | 0 | 0 | 0 | 2 |
| Lamiinae | Ceroplesini | Thysia wallichii tonkinensis (Kreische, 1924) | Asia | 0 | 1 | 0 | 0 | 0 | 1 |
| Lamiinae | Desmiphorini | Anaesthetis testacea (Fabricius, 1781) | Europe | 17 | 0 | 0 | 0 | 0 | 17 |
| Lamiinae | Desmiphorini | Deroplia genei (Aragona, 1830) | Europe | 1 | 0 | 0 | 0 | 0 | 1 |
| Lamiinae | Desmiphorini | Deroplia troberti (Mulsant, 1843) | Europe | 3 | 0 | 0 | 0 | 0 | 3 |
| Lamiinae | Desmiphorini | Eupogonius pauper LeConte, 1852 | North America | 0 | 0 | 1 | 0 | 0 | 1 |
| Lamiinae | Desmiphorini | Eupogonius tomentosus (Haldeman, 1847) | North America | 0 | 0 | 3 | 0 | 0 | 3 |
| Lamiinae | Desmiphorini | Psenocerus supernotatus (Say, 1824) | North America | 0 | 0 | 9 | 0 | 0 | 9 |
| Lamiinae | Dorcaschematini | Dorcaschema cinereum (Olivier, 1800) | North America | 0 | 0 | 1 | 0 | 0 | 1 |
| Lamiinae | Dorcaschematini | Olenecamptus bilobus (Fabricius, 1801) | Asia | 0 | 3 | 0 | 0 | 0 | 3 |
| Lamiinae | Exocentrini | Exocentrus adspersus Mulsant, 1846 | Europe | 5 | 0 | 0 | 0 | 0 | 5 |
| Lamiinae | Exocentrini | Exocentrus lusitanus (Linnaeus, 1767) | Europe | 29 | 0 | 0 | 0 | 0 | 29 |
| Lamiinae | Exocentrini | Exocentrus punctipennis Mulsant & Guillebeau, 1856 | Europe | 28 | 0 | 0 | 0 | 0 | 28 |
| Lamiinae | Lamiini | Lamiomimus gottschei Kolbe, 1886 | Asia | 0 | 1 | 0 | 0 | 0 | 1 |
| Lamiinae | Lamiini | Pharsalia subgemmata (Thomson, 1857) | Asia | 0 | 375 | 0 | 0 | 0 | 375 |
| Lamiinae | Mesosini | Mesosa curculionoides (Linnaeus 1761) | Europe | 37 | 0 | 0 | 0 | 0 | 37 |
| Lamiinae | Mesosini | Mesosa myops (Dalman, 1817) | Asia | 0 | 29 | 0 | 0 | 0 | 29 |
| Lamiinae | Mesosini | Mesosa nebulosa (Fabricius, 1781) | Europe | 132 | 0 | 0 | 0 | 0 | 132 |
| Lamiinae | Monochamini | Anoplophora beryllina (Hope, 1840) | Asia | 0 | 1 | 0 | 0 | 0 | 1 |
| Lamiinae | Monochamini | Anoplophora chinensis (Forster, 1771) | Asia | 0 | 1 | 0 | 0 | 0 | 1 |
| Lamiinae | Monochamini | Anoplophora glabripennis (Motschulsky, 1854) | Asia | 0 | 9 | 0 | 0 | 0 | 9 |
| Lamiinae | Monochamini | Microgoes oculatus (LeConte, 1862) | North America | 0 | 0 | 3 | 0 | 0 | 3 |
| Lamiinae | Monochamini | Monochamus alternatus Hope, 1842 | Asia | 0 | 1246 | 0 | 0 | 0 | 1246 |
| Lamiinae | Monochamini | Monochamus bimaculatus Gahan, 1888 | Asia | 0 | 1 | 0 | 0 | 0 | 1 |
| Lamiinae | Monochamini | Monochamus carolinensis (Olivier, 1797) | North America | 0 | 0 | 77 | 0 | 0 | 77 |
| Lamiinae | Monochamini | Monochamus galloprovincialis (Olivier, 1800) | Europe/Asia | 6209 | 87 | 0 | 0 | 0 | 6296 |
| Lamiinae | Monochamini | Monochamus maculosus Haldeman, 1847 | North America | 0 | 0 | 3 | 0 | 0 | 3 |
| Lamiinae | Monochamini | Monochamus notatus (Drury, 1773) | North America | 0 | 0 | 256 | 0 | 0 | 256 |
| Lamiinae | Monochamini | Monochamus saltuarius Gebler, 1830 | Asia/Europe | 13 | 985 | 0 | 0 | 0 | 998 |
| Lamiinae | Monochamini | Monochamus sartor (Fabricius, 1787) | Europe | 20 | 0 | 0 | 0 | 0 | 20 |
| Lamiinae | Monochamini | Monochamus sartor urussovii (Fischer von Waldheim, 1806) | Asia/Europe | 1 | 41 | 0 | 0 | 0 | 42 |
| Lamiinae | Monochamini | Monochamus scutellatus (Say, 1824) | North America | 0 | 0 | 216 | 0 | 0 | 216 |
| Lamiinae | Monochamini | Monochamus sutor (Linnaeus, 1758) | Europe/Asia | 30 | 22 | 0 | 0 | 0 | 52 |
| Lamiinae | Monochamini | Monochamus sutor longulus Pic, 1898 | Asia | 0 | 22 | 0 | 0 | 0 | 22 |
| Lamiinae | Monochamini | Uraecha angusta (Pascoe, 1857) | Asia | 0 | 15 | 0 | 0 | 0 | 15 |
| Lamiinae | Obereini | Oberea linearis (Linnaeus, 1761) | Europe | 8 | 0 | 0 | 0 | 0 | 8 |
| Lamiinae | Parmenini | Mesolita sp. | Australasia | 0 | 0 | 0 | 3 | 0 | 3 |
| Lamiinae | Parmenini | Parmena balteus (Linnaeus, 1767) | Europe | 7 | 0 | 0 | 0 | 0 | 7 |
| Lamiinae | Parmenini | Parmena unifasciata (Rossi, 1790) | Europe | 3 | 0 | 0 | 0 | 0 | 3 |
| Lamiinae | Phytoeciini | Phytoecia pustulata (Schrank von Paula, 1776) | Europe | 1 | 0 | 0 | 0 | 0 | 1 |
| Lamiinae | Phytoeciini | Phytoecia nigricornis (Fabricius, 1782) | Europe | 2 | 0 | 0 | 0 | 0 | 2 |
| Lamiinae | Pogonocherini | Pogonocherus caroli Mulsant, 1862 | Europe | 5 | 0 | 0 | 0 | 0 | 5 |
| Lamiinae | Pogonocherini | Pogonocherus decoratus Fairmaire, 1855 | Europe | 139 | 0 | 0 | 0 | 0 | 139 |
| Lamiinae | Pogonocherini | Pogonocherus fasciculatus (Degeer, 1775) | Europe | 16 | 1 | 0 | 0 | 0 | 17 |
| Lamiinae | Pogonocherini | Pogonocherus hispidulus (Piller & Mitterpacher, 1783) | Europe | 6 | 0 | 0 | 0 | 0 | 6 |
| Lamiinae | Pogonocherini | Pogonocherus hispidus (Linnaeus, 1758) | Europe | 55 | 0 | 0 | 0 | 0 | 55 |
| Lamiinae | Pogonocherini | Pogonocherus mixtus Haldeman, 1847 | North America | 0 | 0 | 8 | 0 | 0 | 8 |
| Lamiinae | Pogonocherini | Pogonocherus ovatus (Goeze, 1777) | Europe | 19 | 0 | 0 | 0 | 0 | 19 |
| Lamiinae | Pogonocherini | Pogonocherus penicillatus LeConte, 1850 | North America | 0 | 0 | 11 | 0 | 0 | 11 |
| Lamiinae | Pogonocherini | Pogonocherus perroudi Mulsant, 1839 | Europe | 127 | 0 | 0 | 0 | 0 | 127 |
| Lamiinae | Pteropliini | Niphona picticornis Mulsant, 1839 | Europe | 127 | 0 | 0 | 0 | 0 | 127 |
| Lamiinae | Pteropliini | Sthenias gracilicornis Gressitt, 1937 | Europe | 0 | 3 | 0 | 0 | 0 | 3 |
| Lamiinae | Saperdini | Menesia bipunctata (Zoubkoff, 1829) | Europe | 2 | 0 | 0 | 0 | 0 | 2 |
| Lamiinae | Saperdini | Paraglenea fortunei (Saunders, 1853) | Asia | 0 | 1 | 0 | 0 | 0 | 1 |
| Lamiinae | Saperdini | Saperda alberti Plavilstshikov, 1915 | Asia | 0 | 1 | 0 | 0 | 0 | 1 |
| Lamiinae | Saperdini | Saperda hosokawai Hasegawa, 2017 | Asia | 0 | 1 | 0 | 0 | 0 | 1 |
| Lamiinae | Saperdini | Saperda octopunctata (Scopoli, 1772) | Europe | 1 | 0 | 0 | 0 | 0 | 1 |
| Lamiinae | Saperdini | Saperda perforata (Pallas, 1773) | Europe | 21 | 0 | 0 | 0 | 0 | 21 |
| Lamiinae | Saperdini | Saperda populnea (Linnaeus, 1758) | Europe | 4 | 0 | 0 | 0 | 0 | 4 |
| Lamiinae | Saperdini | Saperda scalaris (Linnaeus, 1758) | Europe | 24 | 0 | 0 | 0 | 0 | 24 |
| Lamiinae | Saperdini | Stenostola dubia (Laicharting, 1784) | Europe | 2 | 0 | 0 | 0 | 0 | 2 |
| Lamiinae | Saperdini | Stenostola ferrea (Schrank von Paula, 1776) | Europe | 28 | 0 | 0 | 0 | 0 | 28 |
| Lepturinae | Lepturini | Alosterna tabacicolor (Degeer, 1775) | Europe | 9 | 0 | 0 | 0 | 0 | 9 |
| Lepturinae | Lepturini | Anastrangalia dubia (Scopoli, 1763) | Europe | 6 | 0 | 0 | 0 | 0 | 6 |
| Lepturinae | Lepturini | Anastrangalia reyi (Heyden, 1889) | Europe | 1 | 0 | 0 | 0 | 0 | 1 |
| Lepturinae | Lepturini | Anastrangalia sanguinolenta (Linnaeus 1761) | Europe | 13 | 0 | 0 | 0 | 0 | 13 |
| Lepturinae | Lepturini | Anastrangalia scotodes continentalis (Plavilstshikov, 1936) | Asia | 0 | 1 | 0 | 0 | 0 | 1 |
| Lepturinae | Lepturini | Anoplodera rufipes (Schaller, 1783) | Europe | 5 | 0 | 0 | 0 | 0 | 5 |
| Lepturinae | Lepturini | Anoplodera sexguttata (Fabricius, 1775) | Europe | 9 | 0 | 0 | 0 | 0 | 9 |
| Lepturinae | Lepturini | Brachyleptura brevis (Kirby, 1837) | North America | 0 | 0 | 1 | 0 | 0 | 1 |
| Lepturinae | Lepturini | Brachyleptura circumdata (Olivier, 1800) | North America | 0 | 0 | 1 | 0 | 0 | 1 |
| Lepturinae | Lepturini | Brachyleptura rubrica (Say, 1824) | North America | 0 | 0 | 1 | 0 | 0 | 1 |
| Lepturinae | Lepturini | Leptura thoracica Creutzer, 1799 | Asia | 0 | 1 | 0 | 0 | 0 | 1 |
| Lepturinae | Lepturini | Leptura aethiops Poda von Neuhaus, 1761 | Europe | 2 | 0 | 0 | 0 | 0 | 2 |
| Lepturinae | Lepturini | Leptura aurulenta Fabricius, 1793 | Europe | 7 | 0 | 0 | 0 | 0 | 7 |
| Lepturinae | Lepturini | Leptura quadrifasciata Linnaeus, 1758 | Europe | 9 | 0 | 0 | 0 | 0 | 9 |
| Lepturinae | Lepturini | Neoalosterna capitata (Newman, 1841) | North America | 0 | 0 | 2 | 0 | 0 | 2 |
| Lepturinae | Lepturini | Pachytodes erraticus (Dalman, 1817) | Europe | 232 | 0 | 0 | 0 | 0 | 232 |
| Lepturinae | Lepturini | Paracorymbia fulva (Degeer, 1775) | Europe | 8 | 0 | 0 | 0 | 0 | 8 |
| Lepturinae | Lepturini | Paracorymbia hybrida (Rey, 1885) | Europe | 1 | 0 | 0 | 0 | 0 | 1 |
| Lepturinae | Lepturini | Pedostrangalia revestita (Linnaeus, 1767) | Europe | 12 | 0 | 0 | 0 | 0 | 12 |
| Lepturinae | Lepturini | Pseudovadonia livida (Fabricius, 1776) | Europe | 5 | 0 | 0 | 0 | 0 | 5 |
| Lepturinae | Lepturini | Rutpela maculata (Poda von Neuhaus, 1761) | Europe | 74 | 0 | 0 | 0 | 0 | 74 |
| Lepturinae | Lepturini | Stenurella nigra (Linnaeus 1758) | Europe | 2 | 0 | 0 | 0 | 0 | 2 |
| Lepturinae | Lepturini | Stenurella bifasciata (Müller, 1776) | Europe | 16 | 0 | 0 | 0 | 0 | 16 |
| Lepturinae | Lepturini | Stenurella septempunctata (Fabricius, 1793) | Europe | 5 | 0 | 0 | 0 | 0 | 5 |
| Lepturinae | Lepturini | Stenurella melanura (Linnaeus 1758) | Europe | 33 | 0 | 0 | 0 | 0 | 33 |
| Lepturinae | Lepturini | Stictoleptura canadensis (Olivier, 1800) | North America | 0 | 0 | 8 | 0 | 0 | 8 |
| Lepturinae | Lepturini | Stictoleptura cordigera (Fueßlins, 1775) | Europe | 203 | 0 | 0 | 0 | 0 | 203 |
| Lepturinae | Lepturini | Stictoleptura erythroptera (Hagenbach, 1822) | Europe | 1 | 0 | 0 | 0 | 0 | 1 |
| Lepturinae | Lepturini | Stictoleptura fontenayi (Mulsant, 1839) | Europe | 1 | 0 | 0 | 0 | 0 | 1 |
| Lepturinae | Lepturini | Stictoleptura maculicornis (Degeer, 1775) | Europe | 2 | 0 | 0 | 0 | 0 | 2 |
| Lepturinae | Lepturini | Stictoleptura rubra (Linnaeus, 1758) | Europe/asia | 11 | 1 | 0 | 0 | 0 | 12 |
| Lepturinae | Lepturini | Stictoleptura scutellata (Fabricius, 1781) | Europe | 29 | 0 | 0 | 0 | 0 | 29 |
| Lepturinae | Lepturini | Stictoleptura succedanea (Lewis, 1879) | Asia | 0 | 1 | 0 | 0 | 0 | 1 |
| Lepturinae | Lepturini | Stictoleptura trisignata (Fairmaire, 1852) | Europe | 7 | 0 | 0 | 0 | 0 | 7 |
| Lepturinae | Lepturini | Strangalepta abbreviata (Germar, 1823) | North America | 0 | 0 | 1 | 0 | 0 | 1 |
| Lepturinae | Lepturini | Strangalia attenuata (Linnaeus 1758) | Europe | 1 | 0 | 0 | 0 | 0 | 1 |
| Lepturinae | Lepturini | Strangalia luteicornis (Fabricius, 1775) | North America | 0 | 0 | 4 | 0 | 0 | 4 |
| Lepturinae | Lepturini | Strophiona nitens (Forster, 1771) | North America | 0 | 0 | 3 | 0 | 0 | 3 |
| Lepturinae | Lepturini | Trachysida mutabilis (Newman, 1841) | North America | 0 | 0 | 4 | 0 | 0 | 4 |
| Lepturinae | Lepturini | Trigonarthris proxima (Say, 1824) | North America | 0 | 0 | 1 | 0 | 0 | 1 |
| Lepturinae | Lepturini | Trigonarthris subpubescens (Kirby, 1837) | North America | 0 | 0 | 3 | 0 | 0 | 3 |
| Lepturinae | Lepturini | Typocerus lunulatus (Swederus, 1787) | Asia | 0 | 1 | 0 | 0 | 0 | 1 |
| Lepturinae | Lepturini | Typocerus velutinus (Olivier, 1800) | North America | 0 | 0 | 1 | 0 | 0 | 1 |
| Lepturinae | Lepturini | Vadonia unipunctata (Fabricius, 1787) | Europe | 2 | 0 | 0 | 0 | 0 | 2 |
| Lepturinae | Oxymirini | Anthophylax cyaneus (Haldeman, 1848) | North America | 0 | 0 | 2 | 0 | 0 | 2 |
| Lepturinae | Oxymirini | Anthophylax viridis LeConte, 1850 | North America | 0 | 0 | 6 | 0 | 0 | 6 |
| Lepturinae | Oxymirini | Oxymirus cursor (Linnaeus, 1758) | Europe | 4 | 0 | 0 | 0 | 0 | 4 |
| Lepturinae | Rhagiini | Anisorus quercus (Götz, 1783) | Europe | 3 | 0 | 0 | 0 | 0 | 3 |
| Lepturinae | Rhagiini | Brachyta interrogationis (Linnaeus, 1758) | Europe | 2 | 0 | 0 | 0 | 0 | 2 |
| Lepturinae | Rhagiini | Carilia virginea (Linnaeus, 1758) | Europe | 2 | 0 | 0 | 0 | 0 | 2 |
| Lepturinae | Rhagiini | Carilia virginea thalassina (Schrank von Paula, 1781) | Asia | 0 | 14 | 0 | 0 | 0 | 14 |
| Lepturinae | Rhagiini | Centrodera decolorata (Harris, 1838) | North America | 0 | 0 | 3 | 0 | 0 | 3 |
| Lepturinae | Rhagiini | Cortodera femorata (Fabricius, 1787) | Europe | 11 | 0 | 0 | 0 | 0 | 11 |
| Lepturinae | Rhagiini | Cortodera flavimana (Waltl, 1838) | Europe | 8 | 0 | 0 | 0 | 0 | 8 |
| Lepturinae | Rhagiini | Cortodera humeralis (Schaller, 1783) | Europe | 99 | 0 | 0 | 0 | 0 | 99 |
| Lepturinae | Rhagiini | Dinoptera collaris (Linnaeus, 1758) | Europe | 3 | 0 | 0 | 0 | 0 | 3 |
| Lepturinae | Rhagiini | Acmaeops marginatus (Fabricius, 1781) | Europe/asia | 3 | 11 | 0 | 0 | 0 | 14 |
| Lepturinae | Rhagiini | Acmaeops pratensis (Laicharting, 1784) | Europe | 10 | 0 | 0 | 0 | 0 | 10 |
| Lepturinae | Rhagiini | Acmaeops proteus (Kirby, 1837) | North America | 0 | 0 | 14 | 0 | 0 | 14 |
| Lepturinae | Rhagiini | Acmaeops septentrionis (C G Thomson, 1866) | Europe/asia | 24 | 28 | 0 | 0 | 0 | 52 |
| Lepturinae | Rhagiini | Acmaeops smaragdulus (Fabricius, 1793) | Europe | 6 | 0 | 0 | 0 | 0 | 6 |
| Lepturinae | Rhagiini | Evodinellus borealis (Gyllenhal, 1827) | Asia | 0 | 2 | 0 | 0 | 0 | 2 |
| Lepturinae | Rhagiini | Gaurotes cyanipennis (Say, 1824) | North America | 0 | 0 | 2 | 0 | 0 | 2 |
| Lepturinae | Rhagiini | Grammoptera abdominalis (Stephens, 1831) | Europe | 31 | 0 | 0 | 0 | 0 | 31 |
| Lepturinae | Rhagiini | Grammoptera ruficornis (Fabricius, 1781) | Europe | 266 | 0 | 0 | 0 | 0 | 266 |
| Lepturinae | Rhagiini | Grammoptera ustulata (Schaller, 1783) | Europe | 56 | 0 | 0 | 0 | 0 | 56 |
| Lepturinae | Rhagiini | Pachyta mediofasciata Pic 1936 | Asia | 0 | 3 | 0 | 0 | 0 | 3 |
| Lepturinae | Rhagiini | Pachyta quadrimaculata (Linnaeus, 1758) | Asia | 0 | 1 | 0 | 0 | 0 | 1 |
| Lepturinae | Rhagiini | Paragaurotes ussuriensis (Blessig, 1873) | Asia | 0 | 1 | 0 | 0 | 0 | 1 |
| Lepturinae | Rhagiini | Pidonia lurida (Fabricius, 1792) | Europe | 3 | 0 | 0 | 0 | 0 | 3 |
| Lepturinae | Rhagiini | Pseudosieversia japonica (Ohbayashi, 1937) | Asia | 0 | 1 | 0 | 0 | 0 | 1 |
| Lepturinae | Rhagiini | Rhagium bifasciatum Fabricius, 1775 | Europe | 20 | 0 | 0 | 0 | 0 | 20 |
| Lepturinae | Rhagiini | Rhagium inquisitor (Linnaeus, 1758) | Holarctic | 524 | 5 | 110 | 0 | 0 | 639 |
| Lepturinae | Rhagiini | Rhagium japonicum Bates, 1884 | Asia | 0 | 21 | 0 | 0 | 0 | 21 |
| Lepturinae | Rhagiini | Rhagium mordax (Degeer, 1775) | Europe | 41 | 0 | 0 | 0 | 0 | 41 |
| Lepturinae | Rhagiini | Rhagium rugipenne Reitter, 1898 | Asia | 0 | 4 | 0 | 0 | 0 | 4 |
| Lepturinae | Rhagiini | Rhagium sycophanta (Schrank von Paula, 1781) | Europe | 32 | 0 | 0 | 0 | 0 | 32 |
| Lepturinae | Rhagiini | Stenocorus cinnamopterus (Randall, 1838) | North America | 0 | 0 | 1 | 0 | 0 | 1 |
| Lepturinae | Rhagiini | Stenocorus meridianus (Linnaeus, 1758) | Europe | 71 | 0 | 0 | 0 | 0 | 71 |
| Necydalinae | Necydalini | Necydalis major Linnaeus 1758 | Europe | 3 | 0 | 0 | 0 | 0 | 3 |
| Necydalinae | Necydalini | Necydalis ulmi (Chevrolat, 1838) | Europe | 1 | 0 | 0 | 0 | 0 | 1 |
| Parandrinae | Parandrini | Neandra brunnea (Fabricius, 1798) | North America | 0 | 0 | 1 | 0 | 0 | 1 |
| Prioninae | Aegosomatini | Aegosoma scabricorne (Scopoli, 1763) | Europe | 33 | 0 | 0 | 0 | 0 | 33 |
| Prioninae | Macrotomini | Prinobius myardi Mulsant, 1842 | Europe | 3 | 0 | 0 | 0 | 0 | 3 |
| Prioninae | Meroscelisini | Tragosoma harrisii LeConte, 1851 | North America | 0 | 0 | 236 | 0 | 0 | 236 |
| Prioninae | Prionini | Dorysthenes sternalis (Fairmaire, 1902) | Asia | 0 | 25 | 0 | 0 | 0 | 25 |
| Prioninae | Prionini | Dorysthenes paradoxus (Faldermann, 1833) | Asia | 0 | 22 | 0 | 0 | 0 | 22 |
| Prioninae | Prionini | Dorysthenes sp. | Asia | 0 | 2 | 0 | 0 | 0 | 2 |
| Prioninae | Prionini | Mesoprionus besikanus (Fairmaire, 1855) | Europe | 46 | 0 | 0 | 0 | 0 | 46 |
| Prioninae | Prionini | Orthosoma brunneum (Forster, 1771) | North America | 0 | 0 | 1 | 0 | 0 | 1 |
| Prioninae | Prionini | Prionus coriarius (Linnaeus, 1758) | Europe | 4112 | 0 | 0 | 0 | 0 | 4112 |
| Prioninae | Prionini | Prionus insularis Motschulsky, 1857 | Asia | 0 | 241 | 0 | 0 | 0 | 241 |
| Prioninae | Prionini | Prionus laticollis (Drury, 1773) | North America | 0 | 0 | 3 | 0 | 0 | 3 |
| Prioninae | Prionini | Prionus sp. | Asia | 0 | 1 | 0 | 0 | 0 | 1 |
| Spondylidinae | Anisarthrini | Alocerus moesiacus (Frivaldszky, 1837) | Europe | 4 | 0 | 0 | 0 | 0 | 4 |
| Spondylidinae | Anisarthrini | Anisarthron barbipes (Schrank von Paula, 1781) | Europe | 19 | 0 | 0 | 0 | 0 | 19 |
| Spondylidinae | Asemini | Arhopalus ferus (Mulsant, 1839) | Europe | 338 | 0 | 0 | 0 | 0 | 338 |
| Spondylidinae | Asemini | Arhopalus rusticus (Linnaeus, 1758) | Europe/Asia | 4264 | 702 | 5 | 0 | 0 | 4971 |
| Spondylidinae | Asemini | Asemum amurense Kraatz, 1879 | Asia | 0 | 5 | 0 | 0 | 0 | 5 |
| Spondylidinae | Asemini | Asemum striatum (Linnaeus, 1758) | Holarctic | 21 | 181 | 289 | 0 | 0 | 491 |
| Spondylidinae | Asemini | Asemum tenuicorne Kraatz, 1879 | Europe | 1 | 0 | 0 | 0 | 0 | 1 |
| Spondylidinae | Asemini | Cephalallus oberthueri Sharp, 1905 | Asia | 0 | 14 | 0 | 0 | 0 | 14 |
| Spondylidinae | Asemini | Cephalallus sp. | Asia | 0 | 3 | 0 | 0 | 0 | 3 |
| Spondylidinae | Asemini | Cephalallus unicolor (Gahan, 1906) | Asia | 0 | 15 | 0 | 0 | 0 | 15 |
| Spondylidinae | Asemini | Cephalocrius syriacus (Reitter, 1895) | Europe | 2024 | 0 | 0 | 0 | 0 | 2024 |
| Spondylidinae | Nothorhinini | Nothorhina punctata (Fabricius, 1798) | Europe | 2 | 0 | 0 | 0 | 0 | 2 |
| Spondylidinae | Saphanini | Oxypleurus nodieri Mulsant, 1839 | Europe | 25 | 0 | 0 | 0 | 0 | 25 |
| Spondylidinae | Spondylidini | Spondylis buprestoides (Linnaeus, 1758) | Europe | 2149 | 8 | 0 | 0 | 0 | 2157 |
| Spondylidinae | Tetropiini | Tetropium castaneum (Linnaeus, 1758) | Europe | 53 | 8 | 0 | 0 | 0 | 61 |
| Spondylidinae | Tetropiini | Tetropium cinnamopterum Kirby, 1837 | North America | 0 | 0 | 2 | 0 | 0 | 2 |
| Spondylidinae | Tetropiini | Tetropium fuscum (Fabricius, 1787) | Europe | 100 | 0 | 0 | 0 | 0 | 100 |
| Spondylidinae | Tetropiini | Tetropium gabrieli Weise, 1905 | Europe | 166 | 0 | 0 | 0 | 0 | 166 |
| Spondylidinae | Tetropiini | Tetropium schwarzianum Casey, 1891 | North America | 0 | 0 | 2 | 0 | 0 | 2 |
| Spondylidinae | Tetropiini | Tetrops praeustus (Linnaeus, 1758) | Europe | 7 | 0 | 0 | 0 | 0 | 7 |
| Spondylidinae | Tetropiini | Tetrops starkii Chevrolat, 1859 | Europe | 23 | 0 | 0 | 0 | 0 | 23 |
| Disteniidae | Disteniini | Elytrimitatrix undata (Fabricius, 1775) | North America | 0 | 0 | 6 | 0 | 0 | 6 |
| Vesperidae | Vesperini | Vesperus conicicollis Fairmaire & Coquerel, 1866 | Europe | 1 | 0 | 0 | 0 | 0 | 1 |
| Vesperidae | Vesperini | Vesperus strepens (Fabricius, 1793) | Europe | 6 | 0 | 0 | 0 | 0 | 6 |
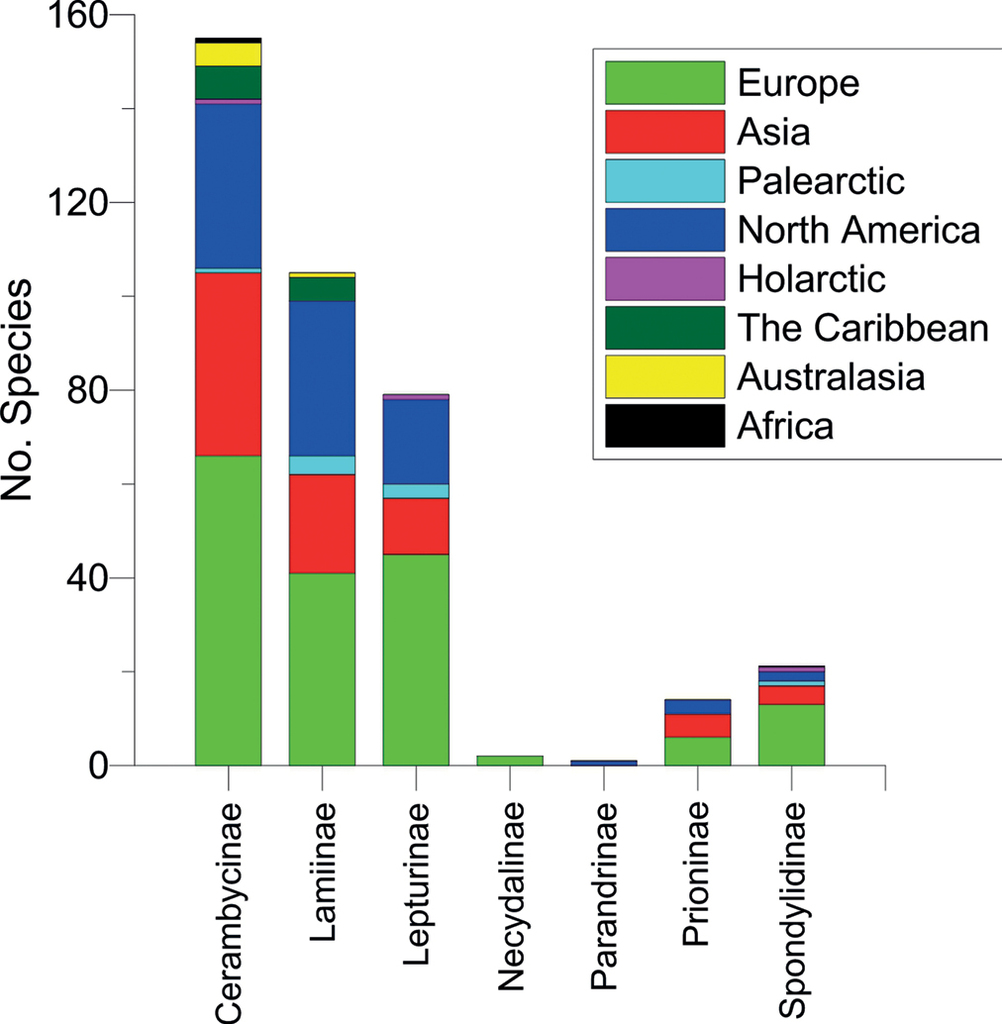
Most tribes included species from the same genera trapped on different continents (Fig.
In Europe, a total of 192 cerambycid species were trapped, of which seven were non-natives (three Clytini: the North American N. a. acuminatus and the Asian X. chinensis and Xylotrechus stebbingi Gahan; three Phoracanthini: the African Cordylomera spinicornis (F.) and the Australasian Phoracantha recurva Newman and P. semipunctata (F.); and one Hesperophanini: the Asian T. campestris). The captures amounted to about 20% of the total European cerambycid fauna (955 species, including apterous species, according to
Three species were notably abundant with captures exceeding > 5,000, including the European native P. testaceus (which was also trapped in the USA as a non-native species), the Palaearctic M. galloprovincialis (trapped in Europe and Northern China) and the Asian X. stebbingi which has invaded Europe. Sixteen species were represented by more than 1,000 specimens, 58 species by more than 100 specimens (Fig.
Some invasive species were trapped in both their native range and in invaded regions (Fig.
Simultaneous captures of non-target Coleopteran species were mostly bark and ambrosia beetles (> 100,000 individuals), which are not yet identified to species, but also predators in the family Cleridae, essentially Clerus mutillarius Fabricius, 1775 (> 5,000 individuals) and Thanasimus spp. (> 2,000 individuals) and Trogossitidae (Temnoscheila spp.; > 500 individuals).
Additional captures resulting from the 10-pheromone blend
The addition of trichoferone and (E)-2-cis-6,7-epoxynonenal to the 8-pheromone blend in France and China in 2019 onwards, did not significantly change the previous trapping spectrum of the 8-pheromone blend (paired t-test; P = 0.750). However, the 10-pheromone blend resulted in trapping large numbers of four Trichoferus species, including the Asian T. campestris in its invasive range in Europe and native range in China (Table
Discussion
Capturing 376 species of cerambycid beetles from eight different subfamilies and 60 tribes on different continents, with 84 species captured in numbers greater than 50 individuals, clearly demonstrates the potential of the multi-pheromone lure to constitute an effective tool for the detection of ‘unexpected’ cerambycid invaders that are accidentally translocated outside their native ranges. Our hypothesis regarding the generic effectiveness of the blend was based on the evolutionary conservatism observed in many cerambycid pheromone structures. Pheromone constituents of the blend composition are shared by phylogenetically-related species on different continents (cf. references in Table
The best represented tribe was Clytini (Cerambycinae). A total of 64 species were trapped overall, including catches in Asia (27 spp.), Europe (22 spp.) and North America (15 spp.). Two of these species were captured in both the native and invaded continents (X. chinensis- Asia/Europe, N. a. acuminatus- North America/Europe). This richness probably resulted from the presence in the blend of C6-ketol (3-hydroxyhexan-2-one) and anti-2,3-hexanediol. Both are known to be male-emitted attractants for a number of species in this tribe (
In the same subfamily Cerambycinae, the tribe Callidiini was represented by 15 species trapped in Europe and five in North America, including a total of 11 species in the genus Phymatodes on the two continents. The very large number of captures (from ~ 2,000 to more than 15,000 individuals) of three Phymatodes species, two native to Europe (P. testaceus and P. alni) and one from North America (P. amoeneus) and those of the closely-related European P. sanguineum, probably reflects the inclusion in the blend of both C6-ketol and 2-methylbutan-1-ol, known to be attractants for a number of Phymatodes spp. (
In the subfamily Lamiinae, large numbers of individuals of 12 species of Monochamini in the genus Monochamus were trapped in Europe, Russia (Siberia), China and North America. This likely resulted from the inclusion in the blend of monochamol (2-[undecyloxy]-ethanol), known as a sex-aggregation pheromone for at least 14 Monochamus species in Europe (M. galloprovincialis;
In the subfamily Spondylidinae,
In the subfamily Prioninae, the inclusion of prionic acid, originally identified as a female-produced sex pheromone of the North American species Prionus californicus Motschulsky (
Despite the general efficiency of our blend, a number of species, especially those trapped with less than 50 individuals, are likely either random catches or were attracted by physical characteristics (e.g. trap shape and/or colour). Based on the previous results of
Attraction of these lepturines may also have been a result of the addition of high release rate ethanol and α-pinene lures to traps, rather than attraction to the blend of synthetic pheromones. Plant volatiles can, in some cases, effectively enhance the attraction of cerambycids to pheromone lures (e.g. for Monochamus species;
Our results also provide leads to possible pheromone structures in new species (see also above), building on the previously-articulated concept of “pheromone identification by proxy”, in which identification of pheromones for one species may provide leads for the identification of pheromones of related taxa (
Trapping of some invasive species in both the native and invaded ranges revealed the potential of the multilure blend for detecting invaders. Some of these non-native species have been present for a long time in the invaded areas (e.g. the European P. testaceus and A. rusticus in North America and the North American N. a. acuminatus in Europe). However, the trapping of very recent invaders within and near ports-of-entry is noteworthy and is indicative of the sensitivity of the blend for early detection at low population levels. For example, the Chinese Clytini X. chinensis was captured in its native range around Beijing, as well as in all the scattered European areas it has invaded and established in relatively recently (2013 in Spain-Catalonia; 2017 in Greece-Crete island and 2018 in southern France-Port of Sète; https://gd.eppo.int/taxon/XYLOCH/distribution/ES). Similarly, when the 10-pheromone blend including trichoferone was deployed, the Chinese Hesperophanini T. campestris was trapped in both its native range in China and in the river port of Huningue (France), where this invasive species had not yet been recorded. Interestingly, despite its presumably low abundance, our trapping studies allowed us to follow the dispersal of this invading species from the port. For example, in 2019 and 2020, specimens were only detected in traps placed within the Huningue Port but, in 2021, the species was captured in traps placed within a 1 km-radius from the Port. Numerous catches of X. stebbingi in ports-of-entry and nurseries of northern France, far from the known invaded southern area of France, also highlighted the sensitivity of the blend for its detection.
What possible improvements can be expected?
Is it possible and useful to continue increasing the number of pheromones included in the blend? The addition of trichoferone and the pheromone of Aromia bungii to the 8-pheromone blend in some field trials in France and China since 2020 resulted in relatively high numbers of captures of several Trichoferus species (three native European species and one native Chinese species invasive in Europe), as well as individuals of A. bungii in China, without reducing the trapping scope observed in nearby traps baited with the primary blend, especially the cerambycine P. testaceus.
For a more general approach of early detection of xylophagous invaders, targeting not only cerambycids, but also other groups, such as bark and ambrosia beetles (Curculionidae, Scolytinae), woodwasps (Siricidae) and jewel beetles (Buprestidae), represents a valuable opportunity. In fact, traps baited with some (e.g.
The position of the trap also has rather to be carefully managed. In our study, standardisation of trap position was not possible due to the different trapping locations (ports-of-entry, urban parks, forests) and the variety of environments amongst the countries included in the study. However, several recent studies have confirmed that trap position can have a considerable influence on the captures of cerambycid beetles, on a vertical gradient from the forest understorey up to the canopy (
Another important point is the colour of the trap. Most traps used in the study were black multifunnel traps (1069 out of 1289; 83%). However,
The impact of such trappings on local insect biodiversity could be questioned. As all specimens from non-target Coleopteran groups have not been identified yet, we cannot exclude that a few species other than cerambycids, bark and ambrosia beetles and beetle predators (clerids, trogossitids) have also been trapped in significant numbers (> 500 ind.). However, any trapping study, like our one, is necessarily limited in scope by cost and logistical factors. Thus, unless trappings are intensively conducted over a whole region or country, which is very unlikely, they are likely to affect local biodiversity in a very limited way.
In conclusion, we are delivering a database of nearly 400 species which were trapped during the course of our multiyear field trials with the multipheromone blend, and the two hypotheses of our study are strongly supported. First, the trapping of a species in significant numbers on a continent effectively increased the probability that it can be detected upon arrival in other countries/continents, as shown by the species trapped in large numbers in both native and invaded ranges, supporting hypothesis 1. Second, the multipheromone blend was shown to be an effective generic attractant for multiple species from several cerambycid subfamilies, including numerous species for which pheromones have not yet been identified, supporting hypothesis 2. In addition, some species, such as the lepturine species caught in large numbers, were probably trapped because of trap colour or the host plant lure, rather than as a result of the blend composition. However, regardless of cues used by beetles, trapping of non-native species when they arrive at ports-of-entry has the same value for phytosanitary officials. Antagonistic effects between compounds exist, but appear to be fairly limited and so should not compromise the overall detection potential. Finally, further advances in the effectiveness of detection of cerambycids by multipheromone lures can be expected as parameters, such as trap colour and height, are optimised and as the number of pheromone components which are found to be conserved within and across related taxa and continents expands.
Acknowledgements
We thank Filippo Giannone, Riccardo Poloni, Kate Van Rooyen, Chantelle Kostanowicz, Vincent Webster, Andrej Kapla, Matic Gabor, Mischa Giasson and Cory Hughes for technical assistance in the lab and field and for species identification. Paige Payter, Michigan State University (MSU), installed and monitored traps in Michigan and Page Payter and Gary Parsons (MSU) identified the captured cerambycids. We are indebted to Fréderic Delport, François-Xavier Saintonge, Jean-Baptiste Daubrée and all colleagues of the “Santé des Forêts” Department (DSF) and local offices (SRAL) of the French Ministry of Agriculture for the management of the traps in France. Marie-Pierre Dufresne from Fredon Centre - Val de Loire and Sylvain Amiot from the Direction Patrimoine végétal et Biodiversité of Tours-Métropole helped to settle traps in the Val de Loire area, France. We are also grateful to Eddy Poirier and Nicolas Moulin for the management of the traps in Martinique and to the forest health team of Vaersa and the forest management service (SOGF) of the Generalitat Valenciana (Spain). We thank very much Xing Zhong-Ping for his help in the trappings in Yunnan and Anastasia Knorre for helping us with field research in the State Nature Reserve “Stolby” (Krasnoyarsk, Russia). We also want to thank Prof. Ana Paula Ramos for enabling a connection with the administration of the municipalities of Lisbon and Setúbal for the trappings in Portugal. We are indebted to Eng. Rui Simão and Eng. Ana Júlia Francisco in CM- Lisboa, Dr. António Nobre from the administration board of Lisbon harbour, Eng. Sérgio Gaspar from CM-Setúbal for allowing us to conduct this work in their municipalities. The municipality of L’Argentière la Bessée provided invaluable assistance for the management of the traps in the southern French Alps. We thank Robert Haack, Nicolas Meurisse and a third anonymous reviewer for their very helpful comments and suggestions on the manuscript.
This work was essentially supported by the HOMED project (HOlistic Management of Emerging Forest Pests and Diseases) which received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No. 771271 (https://homed-projecteu/). We also acknowledge funding from the European project LIFE SAMFIX (SAving Mediterranean Forests from Invasions of Xylosandrus Beetles and associated Pathogenic Fungi, LIFE17 NAT/IT/000609, https://wwwlifesamfixeu/), from the EUPHRESCO project MULTITRAP (“Multi-lure and multi-trap surveillance for invasive tree pests”). Trappings in France were supported from funding by the French Ministry of Agriculture under the PORTRAP project “Test de l’efficacité de pièges génériques multicomposés pour la détection précoce d’insectes exotiques xylophages dans les sites potentiels d’entrée sur le territoire national” and by the Région Centre- Val de Loire under the CANOPEE project (“Biodiversité des insectes des canopées de chênes dépérissants“- Project No. 2018-00124136). Trapping in Austria was supported by the Austrian Federal Ministry for Agriculture, Forestry, Regions and Water Management (Research Project 101183). Trapping in China was partially supported by a grant from the French Embassy in Beijing under the programme CaiYuanPei. LR, JS, YQL and YY are grateful for funding by the National Key Research and Development Program of China (Grant No 2021YFC2600400) and the National Natural Science Foundation of China (31770687). Research conducted in Nova Scotia, Canada, was funded by the Pest Risk Management Program of Natural Resources Canada, Canadian Forest Service. Trapping in Michigan was supported by a grant from the Michigan Department of Agriculture and Rural Development. Research in Siberia (Russia) was supported by Sukachev Institute of Forest SB RAS (the basic project, grant No 0287-2021-0011) [field collection] and the Russian Science Foundation (grant No 22-16-00075) [species identification]. AMR and EKLF are grateful for the Robert Borcer Endowment and the Undergraduate Research Fund of Xavier University and USDA-APHIS cooperative agreement numbers AP19PPQS and T00C082 and AP20PPQS and T00C173. MZ and CM acknowledge funding by the New South Wales government and Hort Innovation project 16004 NSW DPI component of the Macadamia Integrated Pest Management. Trials in Martinique were part of a natural site inventory funded by the DEAL Martinique (French Ministry of Environment). The work in Slovenia was financially supported by the Slovenian Research Agency (Research Core Funding P1-0255). Trapping in England was supported by the Department for Environment, Food and Rural Affairs (Defra). JGM and LMH gratefully acknowledge support from United States Department of Agriculture, Animal and Plant Health Inspection Service (APHIS) grants 19- to 22-8130-1422-CA.
References
- Allison JD, McKenney JL, Millar JG, McElfresh JS, Mitchell RF, Hanks LH (2012) Response of the woodborers Monochamus carolinensis and Monochamus titillator to known cerambycid pheromones in the presence and absence of the host plant volatile α-pinene. Environmental Entomology 41: 1587–1596. https://doi.org/10.1603/EN12185
- Allison JD, Strom B, Sweeney J, Mayo P (2019) Trap deployment along linear transects perpendicular to forest edges: impact on capture of longhorned beetles (Coleoptera: Cerambycidae). Journal of Pest Science 92(1): 299–308. https://doi.org/10.1007/s10340-018-1008-7
- Alvarez G, Gallego D, Hall DR, Jactel H, Pajares GA (2016) Combining pheromone and kairomones for effective trapping of the pine sawyer beetle Monochamus galloprovincialis. Journal of Applied Entomology 140(1–2): 58–71. https://doi.org/10.1111/jen.12297
- Aukema JE, McCullough DG, Von Holle B, Liebhold AM, Britton K, Frankel SJ (2010) Historical accumulation of nonindigenous forest pests in the continental US. BioScience 60: 886–897. https://doi.org/10.1525/bio.2010.60.11.5
- Barbour JD, Millar JG, Rodstein J, Ray AM, Alston DG, Rejzek M, Dutcher JD, Hanks LM (2011) Synthetic 3,5-dimethyldodecanoic acid serves as a general attractant for multiple species of Prionus (Coleoptera: Cerambycidae). Annals of the Entomological Society of America 104(3): 588–593. https://doi.org/10.1603/AN10182
- Bobadoye B, Torto B, Fombong A, Zou Y, Adlbauer K, Hanks LM, Millar JG (2019) Evidence of aggregation-sex pheromone use by longhorned beetle (Coleoptera: Cerambycidae) species native to Africa. Environmental Entomology 48(1): 189–192. https://doi.org/10.1093/ee/nvy164
- Boone CK, Sweeney J, Silk PJ, Hughes CC, Webster RP, Stephen F, MacLauchlan L, Bentz B, Drumont A, Zhao B, Berkvens N, Casteels H, Gregoire JC (2018) Monochamus species from different continents can be effectively detected with the same trapping protocol. Journal of Pest Science 92(1): 3–11. https://doi.org/10.1007/s10340-018-0954-4
- Brockerhoff EG, Liebhold AM (2017) Ecology of forest insect invasions. Biological Invasions 19(11): 3141–3159. https://doi.org/10.1007/s10530-017-1514-1
- Brockerhoff EG, Jones DC, Kimberley MO, Suckling DM, Donaldson T (2006) Nationwide survey for invasive wood-boring and bark beetles (Coleoptera) using traps baited with pheromones and kairomones. Forest Ecology and Management 228(1–3): 234–240. https://doi.org/10.1016/j.foreco.2006.02.046
- Cavaletto G, Faccoli M, Marini L, Spaethe J, Magnani G, Rassati D (2020) Effect of trap color on captures of bark-and wood-boring beetles (Coleoptera; Buprestidae and Scolytinae) and associated predators. Insects 11(11): 749. https://doi.org/10.3390/insects11110749
- Cavaletto G, Faccoli M, Marini L, Spaethe J, Giannone F, Moino S, Rassati D (2021) Exploiting trap color to improve surveys of longhorn beetles. Journal of Pest Science 94(3): 871–888. https://doi.org/10.1007/s10340-020-01303-w
- Collignon RM, Swift IP, Zou Y, McElfresh JS, Hanks LM, Millar JG (2016) The influence of host plant volatiles on the attraction of longhorn beetles (Coleoptera: Cerambycidae) to pheromones. Journal of Chemical Ecology 42: 215–229. https://doi.org/10.1007/s10886-016-0679-x
- Dang Y, Wei K, Wang X, Duan JJ, Jennings DE, Poland TM (2022) Introduced plants induce outbreaks of a native pest and facilitate invasion in the plants’ native range: Evidence from the emerald ash borer. Journal of Ecology 110(3): 593–604. https://doi.org/10.1111/1365-2745.13822
- Eschen R, Holmes T, Smith D, Roques A, Santini S, Kenis M (2014) Likelihood of establishment of tree pests and diseases based on their worldwide occurrence as determined by hierarchical cluster analysis. Forest Ecology and Management 315: 103–111. https://doi.org/10.1016/j.foreco.2013.12.021
- Eschen R, Roques A, Santini A (2015) Taxonomic dissimilarity in patterns of interception and establishment of alien arthropods, nematodes and pathogens affecting woody plants in Europe. Diversity & Distributions 21(1): 36–45. https://doi.org/10.1111/ddi.12267
- Essl F, Bacher S, Blackburn TM, Booy O, Brundu G, Brunel S, Cardoso AC, Eschen R, Gallardo B, Galil B, García-Berthou E, Genovesi P, Groom Q, Harrower C, Hulme PE, Katsanevakis S, Kenis M, Kühn I, Kumschick S, Martinou AF, Nentwig W, O’Flynn C, Pagad S, Pergl J, Pyšek P, Rabitsch W, Richardson DM, Roques A, Roy HE, Scalera R, Schindler S, Seebens H, Vanderhoeven S, Vilà M, Wilson JRU, Zenetos A, Jeschke JM (2015) Crossing frontiers in tackling pathways of biological invasions. Bioscience 65(8): 769–782. https://doi.org/10.1093/biosci/biv082
- Eyre D, Haack RA (2017) Invasive cerambycid pests and biosecurity measures. In: Wang Q (Ed.) Cerambycidae of the world: biology and pest management. CRC Press, Boca Raton, 563–618.
- Fan JT, Denux O, Courtin C, Bernard A, Javal M, Millar JG, Hanks LM, Roques A (2019) Multipheromone blends for trapping native and exotic longhorn beetles at potential points-of-entry and in forests. Journal of Pest Science 92(1): 281–297. https://doi.org/10.1007/s10340-018-0997-6
- Flaherty L, Gutowski JMG, Hughes C, Mayo P, Mokrzycki T, Pohl G, Silk P, van Rooyen K, Sweeney J (2019) Pheromone-enhanced lure blends and multiple trap heights improve detection of bark and wood-boring beetles potentially moved in solid wood packaging. Journal of Pest Science 92(1): 309–325. https://doi.org/10.1007/s10340-018-1019-4
- Graham EE, Mitchell RF, Reagel PF, Barbour JD, Millar JG, Hanks LM (2010) Treating panel traps with a fluoropolymer enhances their efficiency in capturing cerambycid beetles. Journal of Economic Entomology 103(3): 641–647. https://doi.org/10.1603/EC10013
- Graham EE, Poland TM, McCullough DG, Millar JG (2012) A comparison of trap type and height for capturing cerambycid beetles (Coleoptera). Journal of Economic Entomology 105(3): 837–846. https://doi.org/10.1603/EC12053
- Haack RA, Britton KO, Brockerhoff EG, Cavey J, Garrett LJ, Kimberley M, Lowenstein F, Nuding A, Olson LJ, Turner J, Vasilaky KN (2014) Effectiveness of the international phytosanitary standard ISPM no. 15 on reducing wood borer infestation rates in wood packaging material entering the United States. PLoS ONE 9(5): e96611. https://doi.org/10.1371/journal.pone.0096611
- Halloran S, Collignon RM, McElfresh JS, Millar JG (2018) Fuscumol and geranylacetone as pheromone components of Californian longhorn beetles (Coleoptera: Cerambycidae) in the subfamily Spondylidinae. Environmental Entomology 47(5): 1300–1305. https://doi.org/10.1093/ee/nvy101
- Hanks LM, Millar JG (2013) Field bioassays of cerambycid pheromones reveal widespread parsimony of pheromone structures, enhancement by host plant volatiles, and antagonism by components from heterospecifics. Chemoecology 23(1): 21–44. https://doi.org/10.1007/s00049-012-0116-8
- Hanks LM, Millar JG (2016) Sex and aggregation-sex pheromones of cerambycid beetles: Basic science and practical applications. Journal of Chemical Ecology 42(7): 631–654. https://doi.org/10.1007/s10886-016-0733-8
- Hanks LM, Millar JG, Mongold-Diers JA, Wong JCH, Meier LR, Reagel PF, Mitchell RF (2012) Using blends of cerambycid beetle pheromones and host volatiles to simultaneously attract a diversity of cerambycid species. Canadian Journal of Forest Research 42(6): 1050–1059. https://doi.org/10.1139/x2012-062
- Hanks LM, Mongold-Diers JA, Atkinson TH, Fierke MK, Ginzel MD, Graham EE, Poland TM, Richards AB, Richardson ML, Millar JG (2018) Blends of pheromones, with and without host plant volatiles, can attract multiple species of cerambycid beetles simultaneously. Journal of Economic Entomology 111(2): 716–724. https://doi.org/10.1093/jee/tox373
- Hanks LM, Mongold-Diers JA, Mitchell RF, Zou Y, Wong JCH, Meier LR, Johnson TD, Millar JG (2019) The role of minor pheromone components in segregating 14 species of longhorned beetles (Coleoptera: Cerambycidae) of the subfamily Cerambycinae. Journal of Economic Entomology 112(5): 2236–2252. https://doi.org/10.1093/jee/toz141
- Hayes RA, Griffiths MW, Nahrung HF, Arnold PA, Hanks LM, Millar JG (2016) Optimizing generic cerambycid pheromone lures for Australian biosecurity and biodiversity monitoring. Journal of Economic Entomology 109(4): 1741–1749. https://doi.org/10.1093/jee/tow100
- Hoch G, Connell J, Roques A (2020) Testing multi-lure traps for surveillance of native and alien longhorn beetles (Coleoptera, Cerambycidae) at ports of entry and in forests in Austria. Management of Biological Invasions 11(4): 677–688. https://doi.org/10.3391/mbi.2020.11.4.04
- Imrei Z, Domingue MJ, Lohonyai Z, Moreira JA, Bálintné Csonka É, Fail J, Csóka G, Hanks LM, Tóth M, Millar JG (2021) Identification of pheromone components of Plagionotus detritus (Coleoptera: Cerambycidae), and attraction of conspecifics, competitors, and natural enemies to the pheromone blend. Insects 12(10): 899. https://doi.org/10.3390/insects12100899
- Lee S, Lee S (2020) Multigene phylogeny uncovers oviposition-related evolutionary history of Cerambycinae (Coleoptera: Cerambycidae). Molecular Phylogenetics and Evolution 145: 106707. https://doi.org/10.1016/j.ympev.2019.106707
- Lee HR, Lee SC, Lee DH, Jung M, Kwon JH, Huh MJ, Kim DS, Lee JE, Park IK (2018) Identification of aggregation-sex pheromone of the Korean Monochamus alternatus (Coleoptera: Cerambycidae) population, the main vector of pine wood nematode. Journal of Economic Entomology 111(4): 1768–1774. https://doi.org/10.1093/jee/toy137
- Liebhold AM, Brockerhoff EG, Garrett L, Parke J, Britton K (2012) Live plant imports, the major pathway for the forest insect and pathogen invasions of the US. Frontiers in Ecology and the Environment 10(3): 135–143. https://doi.org/10.1890/110198
- Lovett GM, Weiss M, Liebhold A, Holmes TP, Leung B, Lambert KF, Orwig DA, Campbell FT, Rosenthal J, McCullough DG, Wildova R, Ayres MA, Canham CD, Foster DR, LaDeau SL, Weldy T (2016) Nonnative forest insects and pathogens in the US: Impacts and policy options. Ecological Applications 26(5): 1437–1455. https://doi.org/10.1890/15-1176
- Marchioro M, Rassati D, Faccoli M, Van Rooyen K, Kostanowicz C, Webster V, Mayo P, Sweeney JD (2020) Maximizing bark and ambrosia beetle (Coleoptera: Curculionidae) catches in trapping surveys for longhorn and jewel beetles. Journal of Economic Entomology 113(6): 2745–2757. https://doi.org/10.1093/jee/toaa181
- Meier LR, Zou Y, Millar JG, Mongold-Diers JA, Hanks LM (2016) Synergism between enantiomers creates species-specific pheromone blends and minimizes cross-attraction for two species of cerambycid beetles. Journal of Chemical Ecology 42(11): 1181–1192. https://doi.org/10.1007/s10886-016-0782-z
- Meier LR, Millar JG, Mongold-Diers JA, Hanks LM (2019) (S)-sulcatol is a pheromone component for two species of cerambycid beetles in the subfamily Lamiinae. Journal of Chemical Ecology 45(5–6): 447–454. https://doi.org/10.1007/s10886-019-01071-7
- Millar JG, Hanks LM (2017) Chemical ecology of cerambycids In: Wang Q (Ed.) Cerambycidae of the world: biology and pest management. CRC Press/Taylor and Francis, Boca Raton, 161–208.
- Millar JG, Mitchell RF, Mongold-Diers JA, Zou Y, Bográn CE, Fierke MK, Ginzel MD, Johnson CW, Meeker JR, Poland TM, Ragenovich IR, Hanks LM (2018) Identifying possible pheromones of cerambycid beetles by field testing known pheromone components in four widely separated regions of the United States. Journal of Economic Entomology 111(1): 252–259. https://doi.org/10.1093/jee/tox312
- Millar JG, Richards AB, Halloran S, Zou YF, Boyd EA, Quigley KN, Hanks LM (2019) Pheromone identification by proxy: identification of aggregation-sex pheromones of North American cerambycid beetles as a strategy to identify pheromones of invasive Asian congeners. Journal of Pest Science 92: 213–220. https://doi.org/10.1007/s10340-018-0962-4
- Millar JG, Zou YF, Barringer L, Hanks LM (2021) Field trials with blends of pheromones of native and invasive cerambycid beetle species. Environmental Entomology 50: 1294–1298 .https://doi.org/10.1093/ee/nvab085
- Miller DR, Allison JD, Crowe CM, Dickinson DM, Eglitis A, Hofstetter RW, Munson AS, Poland TM, Reid LS, Steed BE, Sweeney JD (2016) Pine sawyers (Coleoptera: Cerambycidae) attracted to α-pinene, monochamol, and ipsenol in North America. Journal of Economic Entomology 109(3): 1205–1214. https://doi.org/10.1093/jee/tow071
- Miller DR, Crowe CM, Mayo PD, Reid LS, Silk PJ, Sweeney JD (2017) Interactions between ethanol, syn-2,3-hexanediol, 3-hydroxyhexan-2-one, and 3-hydroxyoctan-2-one lures on trap catches of hardwood longhorn beetles in Southeastern United States. Journal of Economic Entomology 110(5): 1119–1128. https://doi.org/10.1093/jee/tox188
- Miller SR, Crowe CM, Sweeney JD (2020) Trap height affects catches of bark and woodboring beetles (Coleoptera: Curculionidae, Cerambycidae) in baited multiple-funnel traps in Southeastern United States. Journal of Economic Entomology 113: 273–280. https://doi.org/10.1093/jee/toz271
- Miller DR, Crowe CM, Mayo PD, Silk PJ, Sweeney JD (2022) Interactions between syn-and anti-2, 3-hexanediol lures on trap catches of woodboring beetles and associates in southeastern United States. Environmental Entomology 51(1): 83–93. https://doi.org/10.1093/ee/nvab111
- Mitchell RF, Graham EE, Wong JC, Reagel PF, Striman BL, Hughes GP, Paschen MA, Ginzel MD, Millar JG, Hanks LM (2011) Fuscumol and fuscumol acetate are general attractants for many species of cerambycid beetles in the subfamily Lamiinae. Entomologia Experimentalis et Applicata 141(1): 71–77. https://doi.org/10.1111/j.1570-7458.2011.01167.x
- Nahrung HF, Carnegie AJ (2021) Border interceptions of forest insects established in Australia: intercepted invaders travel early and often. NeoBiota 64: 69–86. https://doi.org/10.3897/neobiota.64.60424
- Nahrung HF, Liebhold AM, Borckerhoff EG, Rassati D (2023) Forest insect biosecurity: Processes, patterns, predictions, pittfalls. Annual Review of Entomology 68(1): 211–229. https://doi.org/10.1146/annurev-ento-120220-010854
- Nie R, Vogler AP, Yang XK, Lin M (2021) Higher-level phylogeny of longhorn beetles (Coleoptera: Chrysomeloidea) inferred from mitochondrial genomes. Systematic Entomology 46: 56–70. https://doi.org/10.1111/syen.12447
- Nolte O, Krieger D (2008) Nachweis von Saperda candida Fabricius 1787 auf Fehmarn – eine weitere, bereits in Ansiedlung befindliche, eingeschleppte Käferart im Mitteleuropa. DgaaE- Nachrichten 22: 133–136. http://wwwdgaaede/html/publi/nachrich/nach22_3pdf
- Pajares JA, Álvarez G, Ibeas F, Gallego D, Hall DR, Farman DI (2010) Identification and field activity of a male-produced aggregation pheromone in the pine sawyer beetle, Monochamus galloprovincialis. Journal of Chemical Ecology 36(6): 570–583. https://doi.org/10.1007/s10886-010-9791-5
- Pajares JA, Alvarez G, Hall DR, Douglas P, Centeno F, Ibarra N, Schroeder M, Teale SA, Wang Z, Yan S, Millar JG, Hanks LM (2013) 2-(Undecyloxy)-ethanol is a major component of the male-produced aggregation pheromone of Monochamus sutor. Entomologia Experimentalis et Applicata 149: 118–127. https://doi.org/10.1111/eea.12113
- Pergl J, Pyšek P, Bacher S, Essl F, Genovesi P, Harrower CA, Hulme PE, Jeschke JM, Kenis M, Kühn I, Perglová I, Rabitsch W, Roques A, Roy DB, Roy HE, Vilà M, Winter M, Nentwig W (2017) Troubling travellers: are ecologically harmful alien species associated with particular introduction pathways? NeoBiota 32: 1–20. https://doiorg/103897/neobiota3210199
- Rabaglia RJ, Cognato AI, Hoebeke ER, Johnson CW, LaBonte JR, Carter ME, Vlach JJ (2019) Early detection and rapid response: A 10-year summary of the USDA Forest Service program of surveillance for non-native bark and ambrosia beetles. American Entomologist 65(1): 29–42. https://doi.org/10.1093/ae/tmz015
- Rassati D, Petrucco Toffolo E, Roques A, Battisti A, Faccoli M (2014) Trapping wood-boring beetles in Italian ports: A pilot study. Journal of Pest Science 87(1): 61–69. https://doi.org/10.1007/s10340-013-0499-5
- Rassati D, Faccoli M, Petrucco Toffolo E, Battisti A, Marini L (2015a) Improving the early detection of alien wood‐boring beetles in ports and surrounding forests. Journal of Applied Ecology 52(1): 50–58. https://doi.org/10.1111/1365-2664.12347
- Rassati D, Faccoli M, Marini L, Haack RA, Battisti A, Petrucco Toffolo E (2015b) Exploring the role of wood waste landfills in early detection of non-native wood-boring beetles. Journal of Pest Science 88(3): 563–572. https://doi.org/10.1007/s10340-014-0639-6
- Rassati D, Marini L, Marchioro M, Rapuzzi P, Magnani G, Poloni R, Di Giovanni F, Mayo P, Sweeney JD (2019) Developing trapping protocols for wood-boring beetles associated with broadleaf trees. Journal of Pest Science 92(1): 267–279. https://doi.org/10.1007/s10340-018-0984-y
- Rassati D, Marchioro M, Flaherty L, Poloni R, Edwards S, Faccoli M, Sweeney JD (2021) Response of native and exotic longhorn beetles to common pheromone components provides partial support for the pheromone-free space hypothesis. Insect Science 28(3): 793–810. https://doi.org/10.1111/1744-7917.12790
- Ray AM, Žunič-Kosi A, Alten RL, McElfresh JS, Hanks LM, Millar JG (2011) cis-Vaccenyl acetate, a female-produced sex pheromone component of Ortholeptura valida, a longhorned beetle in the subfamily Lepturinae. Journal of Chemical Ecology 37: 173–178. DOI 10.1007/s10886-011-9908-5
- Ray AM, Francese JA, Zou Y, Watson K, Crook DJ, Millar JG (2019) Isolation and identification of a male-produced aggregation-sex pheromone for the velvet longhorned beetle, Trichoferus campestris (Faldermann). Scientific Reports 9(1): 4459. https://doi.org/10.1038/s41598-019-41047-x
- Ray AM, Arnold RA, Swift I, Schapker PA, McCann S, Marshall CJ, McElfresh JS, Millar JG (2014) (R)-Desmolactone is a sex pheromone or sex attractant for the endangered valley elderberry longhorn beetle Desmocerus californicus dimorphus and several congeners (Cerambycidae: Lepturinae). PLoS ONE 9(12): e115498. https://doi.org/10.1371/journal.pone.0115498
- Reddy GVP, Fettköther R, Noldt U, Dettner K (2005) Enhancement of attraction and trap catch of the old–house borer, Hylotrupes bajulus (Coleoptera: Cerambycidae), by combination of male sex pheromone and monoterpenes. Pest Management News 61: 699–704. https://doi.org/10.1002/ps.1044
- Rodstein J, McElfresh JS, Barbour JD, Ray AM, Hanks LM, Millar JG (2009) Identification and synthesis of a female-produced sex pheromone for the cerambycid beetle Prionus californicus. Journal of Chemical Ecology 35(5): 590–600. https://doi.org/10.1007/s10886-009-9623-7
- Roques A (2010) Alien forest insects in a warmer world and a globalized economy: Impacts of changes in trade, tourism and climate on forest biosecurity. New Zealand Journal of Forestry 40: 77–94.
- Roques A, Auger-Rozenberg M-A, Blackburn TM, Garnas JR, Pyšek P, Rabitsch W, Richardson DM, Wingfield MJ, Liebhold AM, Duncan RP (2016) Temporal and interspecific variation in rates of spread for insect species invading Europe during the last 200 years. Biological Invasions 18(4): 907–920. https://doi.org/10.1007/s10530-016-1080-y
- Roques A, Shi J, Auger-Rozenberg MA, Ren L, Augustin S, Luo YQ (2020) Are invasive patterns of non-native insects related to woody plants differing between Europe and China? Frontiers in Forests and Global Change 2: 91. https://doi.org/10.3389/ffgc.2019.00091
- Rossa R, Goczał J (2021) Global diversity and distribution of longhorn beetles (Coleoptera: Cerambycidae). The European Zoological Journal 88(1): 289–302. https://doi.org/10.1080/24750263.2021.1883129
- Russo E, Nugnes F, Vicinanza F, Garonna AP, Bernardo U (2020) Biological and molecular characterization of Aromia bungii (Faldermann, 1835) (Coleoptera: Cerambycidae), an emerging pest of stone fruits in Europe. Scientific Reports 10(1): 7112. https://doi.org/10.1038/s41598-020-63959-9
- Ryall K, Silk P, Webster RP, Gutowski JM, Meng Q, Li Y, Gao W, Fidgen J, Kimoto T, Scarr T, Mastro V, Sweeney JD (2015) Further evidence that monochamol is attractive to Monochamus (Coleoptera: Cerambycidae) species, with attraction synergised by host plant volatiles and bark beetle (Coleoptera: Curculionidae) pheromones. Canadian Entomologist 147(5): 564–579. https://doi.org/10.4039/tce.2014.67
- Sarto i Monteys V, Torras i Tutusaus G (2018) A new alien invasive longhorn beetle, Xylotrechus chinensis (Cerambycidae), is infesting mulberries in Catalonia (Spain). Insects 9(2): 52. https://doi.org/10.3390/insects9020052
- Schroeder M, Cocos D, Johansson H, Sweeney J (2021) Attraction of the cerambycid beetles Tetropium gabrieli, T. castaneum and T. fuscum to pheromones and host tree volatiles. Agricultural and Forest Entomology 23(2): 203–211. https://doi.org/10.1111/afe.12422
- Seebens H, Blackburn TM, Dyer EE, Genovesi P, Hulme PE, Jeschke JM, Pagad S, Pyšek P, Winter M, Arianoutsou M, Bacher S, Blasius B, Brundu G, Capinha C, Celesti-Grapow L, Dawson W, Dullinger S, Fuentes N, Jäger H, Kartesz J, Kenis M, Kreft H, Kühn I, Lenzner B, Liebhold A, Mosena A, Moser D, Nishino M, Pearman D, Pergl J, Rabitsch W, Rojas-Sandoval J, Roques A, Rorke S, Rossinelli S, Roy HE, Scalera R, Schindler S, Štajerová K, Tokarska-Guzik B, Van Kleunen M, Walker K, Weigelt P, Yamanaka T, Essl F (2017) No saturation in the accumulation of alien species worldwide. Nature Communications 8(1): 71314435. https://doi.org/10.1038/ncomms14435
- Seebens A, Blackburn TM, Dyer EE, Genovesi P, Hulme PE, Jeschke JM, Pagad S, Pyšek P, Van Kleunen M, Winter M, Ansong M, Arianoutsou M, Bacher S, Blasius B, Brockerhoff EG, Brundu G, Capinha C, Causton CE, Celesti-Grapow L, Dawson W, Dullinger S, Economo EV, Fuentes N, Guénard B, Jäger H, Kartesz J, Kenis M, Kühn I, Lenzner B, Liebhold A, Mosena A, Moser D, Nentwig W, Nishino M, Pearman D, Pergl J, Rabitsch W, Rojas-Sandoval J, Roques A, Rorke S, Rossinelli S, Roy HE, Scalera R, Schindler S, Štajerová K, Tokarska-Guzik B, Walker K, Ward DF, Yamanaka T, Essl F (2018) Global rise in emerging alien species results from increased accessibility of new source pools. Proceedings of the National Academy of Sciences of the USA 115(10): E2264–E2273. https://doiorg/101073/pnas1719429115
- Seebens H, Bacher S, Blackburn TM, Capinha C, Dawson W, Dullinger S, Genovesi P, Hulme PE, van Kleunen M, Kühn I, Jeschke JM, Lenzner B, Liebhold AM, Pattison Z, Pergl J, Pyšek P, Winter M, Essl F (2021) Projecting the continental accumulation of alien species through to 2050. Global Change Biology 27: 970–982. https://doi.org/10.1111/gcb.15333
- Silva WD, Zou Y, Bento JMS, Hanks LM, Millar JG (2017) Aggregation-sex pheromones and likely pheromones of 11 South American cerambycid beetles, and partitioning of pheromone channels. Frontiers in Ecology and Evolution 5: 101. https://doi.org/10.3389/fevo.2017.00101
- Silva WD, Hanks LM, Alvarez JCS, Madalon FZ, Bento JMS, Bello JE, Millar JG (2020) Variations on a theme: Two structural motifs create species-specific pheromone channels for multiple species of South American cerambycid beetles. Insects 11(4): 222. https://doi.org/10.3390/insects11040222
- Sweeney JD, Silk PJ, Grebennikov V (2014) Efficacy of semiochemical-baited traps for detection of longhorn beetles (Coleoptera: Cerambycidae) in the Russian Far East. European Journal of Entomology 111(3): 397–406. https://doi.org/10.14411/eje.2014.049
- Sweeney JD, Hughes C, Webster V, Kostanowicz C, Webster R, Mayo P, Allison JD (2020) Impact of horizontal edge–interior and vertical canopy–understory gradients on the abundance and diversity of bark and woodboring beetles in survey traps. Insects 11(9): 573. https://doi.org/10.3390/insects11090573
- Tavakilian GL, Chevillotte H (2022) Titan: base de données internationales sur les Cerambycidae ou Longicornes.
- Touroult J, Poirier E (2021) Nouvelles espèces et nouveaux signalements de longicornes des Petites Antilles (Coleoptera, Cerambycidae). Bulletin de la Société Entomologique de France 126(1): 15–24. https://doi.org/10.32475/bsef_2161
- Venette RC, Hutchison WD (2021) Invasive insect species: Global challenges, strategies and opportunities. Frontiers in Insect Science 1: 650520. https://doi.org/10.3389/finsc.2021.650520
- Vitali F, Schmitt T (2017) Ecological patterns strongly impact the biogeography of western Palaearctic longhorn beetles (Coleoptera: Cerambycoidea). Organisms, Diversity and Evolution 17: 163–180. https://doi.org/10.1007/s13127-016-0290-6
- Wermelinger B, Flückiger PF, Obrist MK, Duelli P (2007) Horizontal and vertical distribution of saproxylic beetles (Col., Buprestidae, Cerambycidae, Scolytinae) across sections of forest edges. Journal of Applied Entomology 131: 104–114. https://doi.org/10.1111/j.1439-0418.2006.01128.x
- Wickham JD, Harrison RD, Lu W, Guo Z, Millar JG, Hanks LM, Chen Y (2014) Generic lures attract cerambycid beetles in a tropical montane rain forest in southern China. Journal of Economic Entomology 107(1): 259–267. https://doi.org/10.1603/EC13333
- Wickham JD, Lu W, Jin T, Peng Z, Guo D, Millar JG, Hanks LM, Chen Y (2016a) Prionic acid: an effective sex attractant for an important pest of sugarcane, Dorysthenes granulosus (Coleoptera: Cerambycidae: Prioninae). Journal of Economic Entomology 109(1): 484–486. https://doi.org/10.1093/jee/tov266
- Wickham JD, Lu W, Long-Wa Z, Chen Y, Zou Y, Hanks LM, Millar JG (2016b) Likely aggregation-sex pheromones of the invasive beetle species Callidiellum villosulum, and the related Asian species Allotraeus asiaticus, Semanotus bifasciatus, and Xylotrechus buqueti (Coleoptera: Cerambycidae). Journal of Economic Entomology 109(5): 2243–2246. https://doi.org/10.1093/jee/tow187
- Wickham JD, Harrison RD, Lu W, Chen Y, Hanks LM, Millar JG (2021) Rapid assessment of cerambycid beetle biodiversity in a tropical rainforest in Yunnan Province, China, using a multicomponent pheromone lure. Insects 12: 277. https://doi.org/10.3390/insects12040277
- Xu T, Yasui H, Teale SA, Fujiwara-Tsujii N, Wickham JD, Fukaya M, Hansen L, Kiriyama S, Hao D, Nakano A, Zhang L, Watanabe T, Tokoro M, Millar JG (2017) Identification of a male-produced sex-aggregation pheromone for a highly invasive cerambycid beetle, Aromia bungii. Scientific Reports 7(1): 7330. https://doi.org/10.1038/s41598-017-07520-1
- Žunič-Kosi A, Zou YF, Hoskovec M, Vrezec A, Stritih N, Millar JG (2017) Novel, male-produced aggregation pheromone of the cerambycid beetle Rosalia alpina, a priority species of European conservation concern. PLoS ONE 12(8): e0183279. https://doi.org/10.1371/journal.pone.0183279
- Žunič-Kosi A, Stritih-Peljhan N, Zou Y, McElfresh JS, Millar JG (2019) A male-produced aggregation sex pheromone of the beetle Arhopalus rusticus (Coleoptera: Cerambycidae, Spondylinae) may be useful in managing this invasive species. Scientific Reports 9: 19570 .https://doi.org/10.1038/s41598-019-56094-7
Supplementary material
Total trapping network
Data type: site description (excel document)