Methods |
|
Corresponding author: Christina A. Murphy ( christina.a.murphy@gmail.com ) Academic editor: Sidinei Magela Thomaz
© 2022 Christina A. Murphy, William Gerth, Travis Neal, Ivan Arismendi.
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation:
Murphy CA, Gerth W, Neal T, Arismendi I (2022) A low-cost, durable, submersible light trap and customisable LED design for pelagic deployment and capture of fish parasite Salmincola sp. copepodids. NeoBiota 73: 1-17. https://doi.org/10.3897/neobiota.73.76515
|
Abstract
Documenting species invasions and assessments of ecological changes depend on detection. Here, we present a simple design of a plankton light trap with specific wavelength LEDs and modifications. We used PVC pipe to create standardised small, rigid, low-cost traps that can be deployed in lentic habitats. With a cost of under $30 US each, including lights and rechargeable batteries, our traps are affordable without the need for disposable chemical lights. These small traps rely on a vacuum to retain contents upon retrieval, eliminating complicated closing mechanisms and allowing bottom entry. Our design includes submersible LED lights that can withstand pressures of at least 5 atm. We expect that the included instructions for underwater light construction and rubber weights using sand may be broadly applicable. However, we designed and field-tested our traps focusing on the detection and capture of the infective copepodid lifestage of a freshwater parasitic copepod, Salmincola californiensis. This lifestage had previously only been observed by rearing in a laboratory setting and is of concern due to continued spread outside of its native range and detrimental impacts on salmonids, especially in freshwater reservoirs. We used a 445–450 nm wavelength LED light for capturing Salmincola copepodids, but the light design can be modified to any readily available LED and heat sink to attract other target organisms. In our case, the overall affordability of the trap and components allowed for the extensive trapping needed to capture and map the occurrence of rarely-observed species and lifestages, such as the copepodids of S. californiensis. In general, increasing the number of traps that can be deployed within or across sites can aid in the spatial comparisons of plankton distributions needed in studies of ecology and species life histories. Light traps may aid in the detection of introduced zooplankton, such as S. californiensis, outside of their native range and associated plankton community changes.
Keywords
lentic, parasitic copepod, pressure, reservoir, Salmincola californiensis, salmon, zooplankton
Introduction
Zooplankton are integral to the ecology of aquatic habitats (
Many plankton species, especially those with strong swimming abilities, may be phototactic in order to regulate their position in a water column (
Light traps may capture species that are absent in other sampling efforts (
To figure out how we might capture S. californiensis copepodids, we conducted extensive laboratory observations and revised the literature about sampling for infectious stages in other groups of parasitic copepods. We noted, as had previous studies for related species, that S. californiensis copepodids were negatively buoyant and frequently rested on the bottom of laboratory tanks outside of active bouts of swimming (
Methods
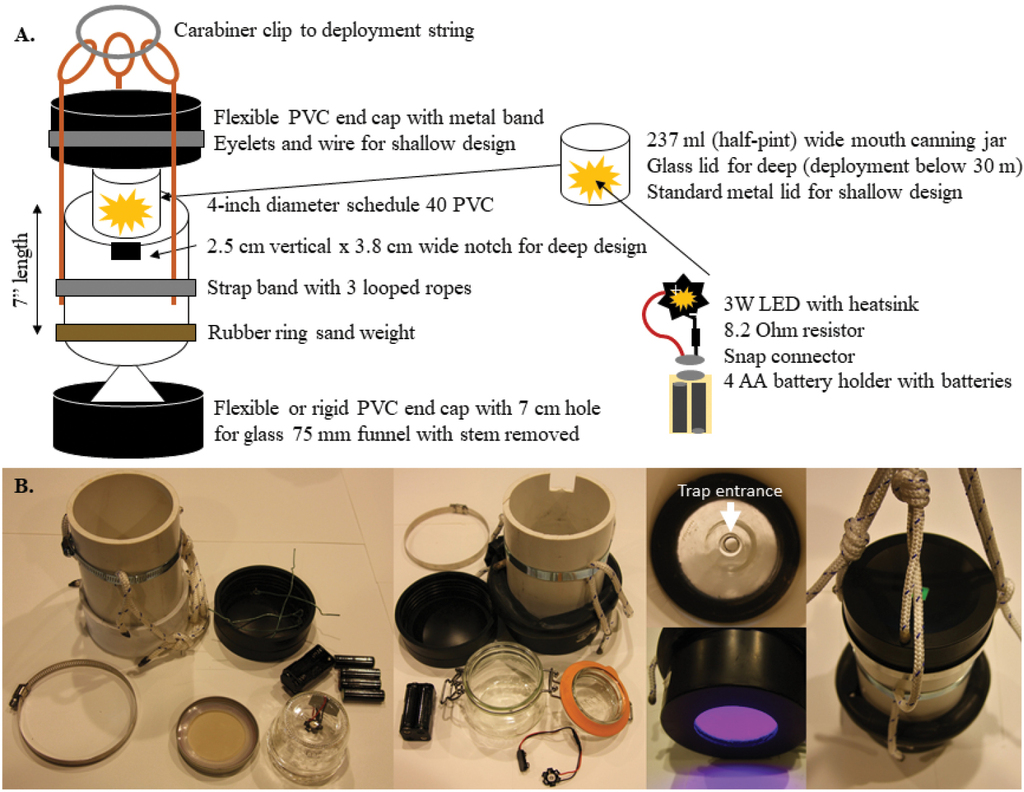
We captured S. californiensis copepodids from the wild (Cougar Reservoir, OR, USA, November and December 2018) using our novel light traps. These first captures of Salmincola sp. relied on prototype traps constructed using clear, glass funnels set into the bases of 5-gallon (19 l) buckets with sealed lids. Although successful, these traps were large and easily damaged. During associated field trials, many of the underwater lights we tried were ineffective, not modifiable and cost prohibitive. Our study sites are used for human recreational purposes and valuable equipment is more likely to be disturbed or removed. Thus, we explored PVC-based traps to focus on improving portability and durability while maintaining a cost that would be reasonable to deploy at a large scale and that could accommodate loss. The final trap design is based on an 18 cm length of 4-inch (10.16 cm) diameter Schedule 40 PVC pipe that is easy to source in the plumbing section of local hardware stores (Suppl. material
Trap design A trap schema and component placement. Light is placed under the lid facing down towards the open inward-facing funnel at the bottom (entrance 8 mm at narrowest point, 75 mm diameter at the widest) B views of traps including standard (left), deep (centre) and funnel, light colour and deployment loops (right). The rubber weight is used on both trap configurations to ensure vertical orientation, but is pictured on the deep trap here, for reference.
Trap assembly
Simple solid selective wavelength LED
We constructed our lights using LEDs that came already affixed to a heatsink. This meant that connections were simple solders of the positive and negative connector wires from the battery snap connectors to the heatsink. We spliced a resistor into the negative wire, although it could be spliced into either wire, and covered the resistor and associated solder connections with heat shrink (Fig.
Optional microcontroller and light array
Our initial trials in the laboratory used lights of differing wavelengths and a microcontroller to adjust intensity and mimic shadows. We describe that version here, although we did not complete field testing using it because solid lights were more effective for our target species during lab comparisons. These instructions should aid in the design of more specialised lights and the code (Supplement) can be adjusted to control individual light parameters. Prototype photos, firmware code and a shopping list for the microcontroller array can be found in Suppl. material
Trap body (PVC cutting, optional notch and securing lines)
For the trap bodies, we cut PVC pipe into ~ 18 cm long pieces using a mitre saw. This was all that was necessary for traps to be deployed in shallow water (< 30 m). For the traps to be used in deep water (≥ 30 m), we cut a 2.5 cm deep by ~ 4 cm wide notch into the top perimeter of those trap bodies. We marked the opening and cut downwards using a hand-held jig-saw. Channel lock pliers were used to bend the newly-created tab of PVC inwards and snap it off. Notches allowed seating of the rear hinges of the snap closures of glass-lidded jars; we wedged the front latch of the closure fully into the trap body when traps were set. Care was taken not to cut the notch too deeply, as the flexible PVC top lid must seal fully below the notch. Further, we prepared traps for deployment by securing three ~ 55 cm lengths of rope to the trap body with a worm gear clamp. We tied a triple sliding hitch in each length of rope so that fine adjustments to vertical balance could be easily made in the field.
Lower lid preparation
When flexible PVC lids were used, they were placed on the PVC pipe to stretch prior to construction to minimise issues during assembly and deployment. Lower lids varied in material, but we took care to minimise the edge spaces where trap contents could become lodged and filled any remaining gaps with silicon for the rigid PVC or ABS lids. This extra care was not necessary with flexible PVC lids. Regardless of bottom lid material, we used a drill press with a 7 cm hole saw to make a perforation in the centre of the lid. We attached the lid to the PVC pipe by either hammering it in place (rigid) or by placing it on and securing it with a worm-gear hose clamp tightened with a screwdriver (flexible PVC).
Funnels were prepared by cutting the stem off (before the opening widened) using a hand-held metal file (scoring the stem on all sides). We then lined the large end of the funnel opening with silicone (for adhesion) and firmly placed the funnel inside on the lower lid of the trap to cover the hole in the lid and ensuring a complete seal around the funnel perimeter. We left the trap open and undisturbed for 24 hours to allow the silicone to cure and the funnel to be fully secured.
Top lid preparation
Top lids were always the type made from flexible PVC. The top lid was used without modification for traps for deep deployment that used glass snap-top jars, because the jars were held at the top of the trap by the notch in the PVC for their closing mechanism. Standard, metal-top jam jars used for traps deployed in shallow water needed to be secured to the top lid. For this, we used four small eyelet screws placed evenly spaced just inside of the ring where the PVC tube is seated when the cap is on. We threaded one wire piece through each screw and secured the wire with pliers. We then bent the wires around each other by hand when loading the light jars during deployment
Weights
We cut used bicycle inner tubes into sections of approximately 55 cm, discarding the stem valve and pieces with large holes. We sealed one open end of the tube by folding it over and securing it with duct tape. Then we used a funnel to fill the tube with sand. We left ~ 8 cm of the tube unfilled and looped the tube around a length of ~ 10 cm diameter PVC so that the open, unfilled section overlapped the sealed end. We then wrapped the two ends together with tape to seal and secure the weight in a circular form of ~ 12.5 cm diameter. The weights stretched, so there was flexibility in sizing. Bike tube diameters also varied, but weights were only used to keep traps orientated vertically, so exact weighting was not necessary.
Deployment line features
To allow setting of multiple traps along a vertical transect, we prepared loops at pre-measured distances along a deployment line of a measured length (we made loops at 1 m, 2.5 m, 5 m, 10 m, 15 m and 20 m along a 25 m line) that was also looped at both ends. Loops at the top and bottom of each line were used for attaching an anchor and a buoy. We ensured that butterfly loops along the line were sized to allow the weighted traps to be positioned next to the line without being forced at an angle (e.g. > 9 cm). Anchors were made by adding water to concrete mix and pouring the concrete into ~ 15 cm lengths of concrete form tube (placed on disposable plastic or cardboard). We placed one eyebolt into each concrete anchor, ensuring the bolt was in place and jiggling the mould after placement to settle the concrete around the bolt. Anchors were left to dry for approximately one week, though curing was weather dependent. Additional lines were prepared in pre-measured spools to link deployment lines to buoys when waterbody depths were greater than the length of the deployment lines.
Trap deployment
We placed a light (facing down) in each jar and secured it into position with a small piece of tape. Then we put batteries into holders and snapped the holders to the light contacts. Lights were placed in either jars sealed with a silicone ring, metal lid and metal band for shallow deployment (< 30 m) or snap-top jars with glass lids and rubber gaskets for deep deployment (≥ 30 m). The glass-lidded jars were needed for deep deployment because metal jar lids crush from the pressure at depths of more than 30 m. Note: batteries should not be added early to unclipped battery holders, especially in metal-lidded jars, to avoid a risk of shorting.
Jars were fixed into position in the traps either by twisting the wires attached to the lid (shallow) or by being seated into the notch cut into the trap body (deep). We filled a tote with filtered water that we used to fill the traps prior to deployment. After filling each trap with water by submerging it in the tote, we secured the top lid to each trap while submerged and used a screwdriver to tighten the associated clamp, making sure that the clamp held the lid tightly to the trap body with a complete seal. We then clipped each trap to a loop in the deployment string as we lowered the string and associated anchor into the water.
Trap collection
At each vertical transect, traps were collected sequentially from surface to bottom and placed into white primary wash basins (11.4 l) upon retrieval. These were labelled with the depth for each trap. Once trap lids were loosened and the vacuum was broken, contents flowed into the small primary basin. Wash bottles were used to thoroughly clean out each trap and wash any additional organisms into the wash basin. The basin contents were then sieved using a 106 µm test sieve and washed through a funnel into a collection bottle (250 ml HDPE Nalgene). Primary basins found to contain juvenile fish were nested inside of larger (17 l) secondary basins containing ice for at least 20 min to anaesthetise the fish. All samples were preserved using 95% ethanol with a final concentration of at least 75%.
Field assessment
We set our light traps monthly from June through to December 2019 at three reservoirs (Cougar, Fall Creek and Lookout Point) in the Upper Willamette Basin, Oregon, USA. From June through to September, we set five vertical strings at each reservoir (Fig.
Representation of field deployment set-up over A space and B depth within reservoirs in the Willamette Basin, Oregon. Traps were placed at five sites per reservoir (as indicated with white circles) with traps at 1, 2.5 and 5 m from the benthos continued by traps at 5 m intervals to the surface along a single vertical deployment string held in place by an anchored buoy. Traps were clipped on to pre-tied loops in the deployment rope (represented as dashed orange ovals) to facilitate placement.
For our field test, each month, we deployed traps for 48-hour and lights continued to operate throughout deployment. We chose to use 445–450 nm wavelength LED lights in our traps because laboratory trials we conducted showed these were better at attracting S. californiensis copepodids than white LEDs or LEDs producing narrow bands of longer or shorter wavelengths (Suppl. material
Field-collected light trap samples were examined in the laboratory as described below. Further, for comparison with light traps results, we also collected monthly vertical tow samples and Van Dorn trap samples at each reservoir (see
Laboratory methods
Light trap samples were processed in the laboratory to identify and count S. californiensis copepodids and other non-target organisms that were also captured. To increase our efficiency at searching for S. californiensis copepodids, we washed each sample through a pair of stacked sieves (Fig.
Photos of sample processing and characteristics of S. californiensis copepods compared to other common zooplankton in light trap samples A stacked sieves used in light trap sample processing B large zooplankton retained on the 500 µm sieve C smaller zooplankton that could include S. californiensis copepodids retained on the 106 µm sieve D freshly preserved S. californiensis copepodids from a laboratory rearing experiment E comparison of common zooplankton caught in a light trap and retained on a 106 µm sieve including: a) a calanoid copepod, b) a cyclopoid copepod, c) a S. californiensis copepodid and d) a Daphnia species.
In practice, distinguishing S. californiensis copepodids from other zooplankton was not difficult. We had examined many of these copepodids during laboratory hatching and rearing experiments (
After searching for and removing S. californiensis copepodids from each light trap sample, we drained the alcohol from the remaining material in the Petri dish using the 106 µm sieve and rinsed this material and the material retained on the mesh of the 500 µm sieve into a beaker with water; a couple drops of detergent were added to prevent organisms from clumping. We then subsampled these non-target organisms using a Folsom plankton splitter to target 200–400 organisms for representative identification and enumeration.
Results
We successfully captured S. californiensis copepodids using our light traps, but did not detect them in concurrent tow or Van Dorn traps samples (Table
Number of light traps that captured Salmincola californiensis copepodids in our field trial at three upper Willamette Basin reservoirs. Samples were collected monthly from June through to December 2019. Number of traps set given in parentheses. Note: corresponding tow net samples from 35 m to surface (0.5 m diameter, 64 µm) failed to capture copepodids during the study period.
| Reservoir | June | July | August | September | October | November | December |
|---|---|---|---|---|---|---|---|
| Cougar | 0 (25) | 1 (29) | 3 (28) | 10 (28) | 7 (33) | 7 (29) | 8 (28) |
| Fall Creek | 0 (23) | 0 (26) | 0 (26) | 8 (28) | 7 (23) | 0* (2*) | 1* (11*) |
| Lookout Point | 0 (24) | 1 (27) | 0 (27) | 0 (28) | 0 (33) | 0 (30) | 0 (24) |
Discussion
Here, we describe light traps that successfully attract and capture Salmincola copepodids from lentic systems with the potential to be used to examine patterns of both spatial and vertical distribution of this species in our study reservoirs. In order to sample for this ‘needle in a haystack’, we present a robust low-cost trap design that can be widely deployed, including at depths with pressures of 5.9 bar and in environments that could damage mesh-based traps (e.g. reservoirs with large stumps and woody debris). The simple design eliminates the need for complex and expensive closing mechanisms and is constructed from easily-obtained materials. By establishing methods to capture this poorly-understood copepodid life stage, we will be able to gain valuable ecological knowledge about its density and distribution over time and space. This information should help us to understand the current problem of high infection prevalence and intensity and may allow us develop remediation strategies. In addition, the use of our light traps provide monitoring data to prompt ecological discoveries (e.g. use of optimised traps as potential method of control and management of copepodids).
We focused on 48-hour deployments because it increases trap captures and allows for us to set strings at multiple sites during our sampling window. Captures are thus possible throughout the day and night periods. Even so, we would expect most captures to happen at night, when the lights provide a greater difference in illumination from background natural levels. We did not focus on new moon periods, though moonlight could modify capture efficiency (
Inexpensive traps can be important to achieve research goals with limited funding. We expect these traps to be especially useful for rare or poorly-understood species where large-scale deployment may be necessary. They are also ideal for areas with boat traffic where traps may be stolen or damaged. The option to deploy traps at depth may provide greater captures, as other studies have found increased catches near benthic areas (
Future studies could explore the efficiency of these traps, especially for other taxa and the changes in capture composition with alternative LEDs and/or differing LED intensities. Blue and green wavelengths are likely ideal for a broad range of taxa and transmit well in water (
Outside of light trapping, we expect that the instructions (Supplement) for underwater light construction and rubber weights may be broadly applicable. Our description of how to assemble LED light sources for use underwater allows for researchers to customise wavelengths and target desired taxa. Low-cost selective wavelength LEDs may also be useful for other underwater applications (e.g. video). Weights that use discarded materials (bike tubes) and sand can aid in balancing equipment underwater where cost or vandalism are of concern. In our case, the overall affordability of the trap and components allows for the extensive trapping needed to capture and map the occurrence of rarely-observed species and lifestages, such as the copepodids of S. californiensis. In general, increasing the number of traps that can be deployed within or across sites can aid in the spatial comparisons of plankton distributions needed in studies of ecology and life history. Effective trapping of zooplankton, parasitic and free-living, may be critical to early detection of invasions or novel ecological dynamics.
Acknowledgements
Thank you to Chee Sing Lee for assistance in LED electronic assembly and design. The authors acknowledge US Army Corps of Engineers, JPL 19-03-SYS, the USGS OR Cooperative Fish and Wildlife Research Unit and Oregon State University for funding and technical support during this research. We especially thank Emilee Mowlds and Erik Swanson for trap construction and field deployment as well as Todd Pierce for trap deployment, recovery, boating and logistics. Laboratory testing would not have been possible without Ryan Koch and the Smith Farm facilities. James Peterson, Carl Shreck, Crystal Herron, Justin Saunders and Mike Kent provided discussion leading to successful trap design. David Noakes and OSU Surplus provided materials and additional inspiration. The views expressed in this publication are solely those of the authors and do not reflect official policy of the U.S. Government nor endorse any products or commercial services.
References
- Beers JR, Stewart GL, Strickland JD (1967) A pumping system for sampling small plankton. Journal of the Fisheries Board of Canada 24(8): 1811–1818. https://doi.org/10.1139/f67-147
- Bron JE (1993) A study of the biology and behaviour of the copepodid larva of the salmon louse Lepeophtheirus salmonis (Kroyer, 1837) (Copepoda: Caligidae). Ph.D. University of Stirling http://hdl.handle.net/1893/625 [August 26, 2019]
- Burkett DA, Lee WJ, Lee KW, Kim HC, Lee HI, Lee JS, Shin EH, Wirtz RA, Cho HW, Claborn DM, Coleman RE, Klen TA (2001) Light carbon dioxide, and octenol-baited mosquito trap and host-seeking activity evaluations for mosquitoes in a malarious area of the Republic of Korea. Journal of the American Mosquito Control Association 17: 196–205.
- De Bernardi R (1984) Methods for the estimation of zooplankton abundance. A manual on methods for the assessment of secondary productivity in fresh waters. IBP Handbook no. 17. Blackwell, Oxford, 59–86.
- Doherty PJ (1987) Light-traps: Selective but useful devices for quantifying the distributions and abundances of larval fishes. Bulletin of Marine Science 41: 423–431.
- Duggan IC, Pullan SG (2017) Do freshwater aquaculture facilities provide an invasion risk for zooplankton hitchhikers? Biological Invasions 19(1): 307–314. https://doi.org/10.1007/s10530-016-1280-5
- Fasten N (1912) The brook-trout disease at Wild Rose and other hatcheries. Biennial Report of the Commissioners of Fisheries of the State of Wisconsin, 12–22.
- Fisher R, Bellwood DR (2002) A light trap design for stratum-specific sampling of reef fish larvae. Journal of Experimental Marine Biology and Ecology 269(1): 27–37. https://doi.org/10.1016/S0022-0981(01)00384-7
- Floyd KB, Courtenay WH, Hoyt RD (1984) A new larval fish light trap: The quatrefoil trap. Progressive Fish-Culturist 46(3): 216–219. https://doi.org/10.1577/1548-8640(1984)46<216:ANLFLT>2.0.CO;2
- Forward RB (1988) Diel vertical migration: Zooplankton photobiology and behaviour. Oceanography and Marine Biology - an Annual Review 26: 1–393.
- Freckelton ML, Nedved BT, Hadfield MG (2017) Induction of invertebrate larval settlement; different bacteria, different mechanisms? Scientific Reports 7(1): e42557. https://doi.org/10.1038/srep42557
- Hargis LN, Lepak JM, Vigil EM, Gunn C (2014) Prevalence and intensity of the parasitic copepod (Salmincola californiensis) on Kokanee salmon (Oncorhynchus nerka) in a reservoir in Colorado. The Southwestern Naturalist 59(1): 126–129. https://doi.org/10.1894/N06-JC-72.1
- Havel JE, Shurin JB (2004) Mechanisms, effects, and scales of dispersal in freshwater zooplankton. Limnology and Oceanography 49(4part2): 1229–1238. https://doi.org/10.4319/lo.2004.49.4_part_2.1229
- Hernandez FJ, Shaw RF (2003) Comparison of plankton net and light trap methodologies for sampling larval and juvenile fishes at offshore petroleum platforms and a coastal jetty off Louisiana. American Fisheries Society Symposium. Citeseer, 15–38.
- Herron CL, Kent ML, Schreck CB (2018) Swimming endurance in juvenile Chinook Salmon infected with Salmincola californiensis. Journal of Aquatic Animal Health 30(1): 81–89. https://doi.org/10.1002/aah.10010
- Heuch PA, Doall MH, Yen J (2007) Water flow around a fish mimic attracts a parasitic and deters a planktonic copepod. Journal of Plankton Research 29(suppl_1): i3–i16. https://doi.org/10.1093/plankt/fbl060
- Hickford M, Schiel D (1999) Evaluation of the performance of light traps for sampling fish larvae in inshore temperate waters. Marine Ecology Progress Series 186: 293–302. https://doi.org/10.3354/meps186293
- Jones DA (1971) A new light trap for plankton. Fourth European Marine Biology Symposium. Cambridge University Press London, 487–493.
- Kabata Z, Cousens B (1973) Life cycle of Salmincola californiensis (Dana 1852) (Copepoda: Lernaeopodidae). Journal of the Fisheries Research Board of Canada 30(7): 881–903. https://doi.org/10.1139/f73-150
- Kamerath M, Allen BC, Chandra S (2009) First documentation of Salmincola californiensis in Lake Tahoe, CA – NV, USA. Western North American Naturalist 69(2): 257–259. https://doi.org/10.3398/064.069.0216
- Kehayias G (2006) A simple and inexpensive light trap for lake zooplankton. Journal of Freshwater Ecology 21(3): 539–541. https://doi.org/10.1080/02705060.2006.9665034
- Kehayias G, Antonou M, Zerva M, Karachalios I (2008) Using plankton nets as light traps: Application with chemical light. Journal of Plankton Research 30(9): 1075–1078. https://doi.org/10.1093/plankt/fbn060
- Lehman JT (1988) Ecological principles affecting community structure and secondary production by zooplankton in marine and freshwater environments1. Limnology and Oceanography 33(4part2): 931–945. https://doi.org/10.4319/lo.1988.33.4part2.0931
- Lepak JM, Hansen AG, Hooten MB, Brauch D, Vigil EM (2021) Rapid proliferation of the parasitic copepod, Salmincola californiensis (Dana), on kokanee salmon, Oncorhynchus nerka (Walbaum), in a large Colorado reservoir. Journal of Fish Diseases. https://doi.org/10.1111/jfd.13539
- Martynova DM, Gordeeva AV (2010) Light-dependent behavior of abundant zooplankton species in the White Sea. Journal of Plankton Research 32(4): 441–456. https://doi.org/10.1093/plankt/fbp144
- McLeod LE, Costello MJ (2017) Light traps for sampling marine biodiversity. Helgoland Marine Research 71(1): 1–8. https://doi.org/10.1186/s10152-017-0483-1
- Meekan M, Wilson S, Halford A, Retzel A (2001) A comparison of catches of fishes and invertebrates by two light trap designs, in tropical NW Australia. Marine Biology 139(2): 373–381. https://doi.org/10.1007/s002270100577
- Monzyk FR, Friesen TA, Romer JD (2015) Infection of juvenile salmonids by Salmincola californiensis (Copepoda: Lernaeopodidae) in reservoirs and streams of the Willamette River Basin, Oregon. Transactions of the American Fisheries Society 144(5): 891–902. https://doi.org/10.1080/00028487.2015.1052558
- Murphy CA, Evans A, Coffin B, Arismendi I, Johnson SL (2019a) Resilience of zooplankton communities in temperate reservoirs with extreme water level fluctuations. Inland Waters 10(2): 256–266. https://doi.org/10.1080/20442041.2019.1657349
- Murphy CA, Taylor G, Pierce T, Arismendi I, Johnson SL (2019b) Short-term reservoir draining to streambed for juvenile salmon passage and non-native fish removal. Ecohydrology 12(6): e2096. https://doi.org/10.1002/eco.2096
- Murphy CA, Gerth W, Arismendi I (2020) Hatching and survival of the salmon ‘gill maggot’ Salmincola californiensis (Copepoda: Lernaeopodidae) reveals thermal dependence and undocumented naupliar stage. Parasitology 147(12): 1338–1343. https://doi.org/10.1017/S0031182020001109
- Neal T, Kent ML, Sanders J, Schreck CB, Peterson JT (2021) Laboratory infection rates and associated mortality of juvenile Chinook Salmon (Oncorhynchus tshawytscha) from parasitic copepod (Salmincola californiensis). Journal of Fish Diseases 44(9): 1423–1434. https://doi.org/10.1111/jfd.13450
- Novales Flamarique I, Gulbransen C, Galbraith M, Stucchi D, Stucchi D (2009) Monitoring and potential control of sea lice using an LED-based light trap. Canadian Journal of Fisheries and Aquatic Sciences 66(8): 1371–1382. https://doi.org/10.1139/F09-094
- Penston MJ, McKibben MA, Hay DW, Gillibrand PA (2004) Observations on open-water densities of sea lice larvae in Loch Shieldaig, western Scotland. Aquaculture Research 35(8): 793–805. https://doi.org/10.1111/j.1365-2109.2004.01102.x
- Penston MJ, Millar CP, Zuur A, Davies IM (2008) Spatial and temporal distribution of Lepeophtheirus salmonis (Krøyer) larvae in a sea loch containing Atlantic salmon, Salmo salar L., farms on the north-west coast of Scotland. Journal of Fish Diseases 31(5): 361–371. https://doi.org/10.1111/j.1365-2761.2008.00915.x
- Porter SS, Eckert GL, Byron CJ, Fisher JL (2008) Comparison of light traps and plankton tows for sampling brachyuran crab larvae in an Alaskan fjord. Journal of Crustacean Biology 28(1): 175–179. https://doi.org/10.1651/06-2818R.1
- Poulin R, Curtis MA, Rau ME (1990) Responses of the fish ectoparasite Salmincola edwardsii (Copepoda) to stimulation, and their implication for host-finding. Parasitology 100(3): 417–421. https://doi.org/10.1017/S0031182000078707
- Suárez-Morales E, Mercado-Salas N (2013) The non-indigenous parasitic copepod Neoergasilus japonicus (Harada) (Cyclopoida) from central Mexico: The earliest invasion in continental America. BioInvasions Records 2(3): 201–206. https://doi.org/10.3391/bir.2013.2.3.05
- Thorp JH, Rogers DC (Eds) (2016) Thorp and Covich’s Freshwater Invertebrates, Volume II: Keys to Nearctic Fauna. 4th edn. Elsevier, New York. https://doi.org/10.1016/B978-0-12-385028-7.09001-6
- Tor A, Deudero S, Carbonell A, Goni R, Stobart B (2009) Coastal meroplanktonic larval stages of Peninsula de Llevant Reserve determined with light traps. Bolletí de la Societat d'Història Natural de les Balears 53: 97–105.
- Tranter DJ, Bulleid NC, Campbell R, Higgins HW, Rowe F, Tranter HA, Smith DF (1981) Nocturnal movements of phototactic zooplankton in shallow waters. Marine Biology 61(4): 317–326. https://doi.org/10.1007/BF00401571
- Watson M, Power R, Simpson S, Munro JL (2002) Low cost light traps for coral reef fishery research and sustainable ornamental fisheries. Naga. The ICLARM Quarterly 25: 4–7.
- Yan ND, Girard R, Boudreau S (2002) An introduced invertebrate predator (Bythotrephes) reduces zooplankton species richness. Ecology Letters 5(4): 481–485. https://doi.org/10.1046/j.1461-0248.2002.00348.x
Supplementary material
Supplementary material for a low-cost, durable, submersible light trap and customizable LED design for pelagic deployment and capture of fish parasite Salmincola sp. copepodids
Data type: Pdf file.
Explanation note: Table S1. List of materials used to construct light traps and deployment strings. Table S2. Laboratory captures in light trap development. Table S3. Additional materials used for microcontroller programmed lights in series. Table S4. Examples of plankton captures categorised as Cladocera, Calanoida, Cyclopoida and Salmincola californiensis for Fall Creek and Cougar Reservoirs in late October 2019. Figure S1. Wiring diagrams. Figure S2. Prototype light configuration with Arduino and firmware code (.ino) for varying the light intensity in series. Manual S1. Pictographic light trap assembly instructions.