Research Article |
|
Corresponding author: Łukasz Wejnerowski ( wejner@amu.edu.pl ) Academic editor: Sidinei Magela Thomaz
© 2022 Łukasz Wejnerowski, Tümer Orhun Aykut, Aleksandra Pełechata, Michał Rybak, Tamara Dulić, Jussi Meriluoto, Marcin Krzysztof Dziuba.
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation:
Wejnerowski Ł, Aykut TO, Pełechata A, Rybak M, Dulić T, Meriluoto J, Dziuba MK (2022) Plankton hitch-hikers on naturalists’ instruments as silent intruders of aquatic ecosystems: current risks and possible prevention. NeoBiota 73: 193-212. https://doi.org/10.3897/neobiota.73.82636
|
Abstract
Organism dispersal is nowadays highly driven by human vectors. This also refers to the aquatic organisms that can often silently spread in and invade new waters, especially when human vectors of dispersal act without brakes. Thus, it is mandatory to continuously identify human-mediated mechanisms of organism dispersal and implement proper biosecurity treatments. In this study, we demonstrate how the plankton net – one of the basic instruments in the equipment of every plankton sampling person is a good vector for plankton dispersal and invasions. We also demonstrate whether keeping the net in an ethanol solution after sampling is a proper biosecurity treatment, and what kind of treatments are implemented by people worldwide. The first simulation shows that bloom-forming cyanobacteria can easily infiltrate into the new environment thanks to the nets, and can prosper there. However, ethanol-based biosecurity treatment efficiently prevented their spread and proliferation in the new environment. The second simulation, based on wild plankton from an eutrophic lake, indicates that a plethora of phyto- and zooplankton taxa can infiltrate into the new waterbody through the net and sustain themselves there if the net is only flushed in the waterbody and left to dry after sampling (an approach that is commonly used by naturalists). Here, we also show that native plankton residents strongly shape the fate of hitch-hikers, but some of them like cyanobacteria can successfully compete with residents. Survey data alert us to the fact that the vast majority of biologists use either ineffective or questionable biosecurity treatments and only less than a tenth of samplers implement treatments based on disinfectant liquids. Our results emphasize that the lack of proper biosecurity methods implemented by the biologists facilitates the spread and invasions of plankton including also invasive species of a great nuisance to native ecosystems. Considering that naturalists usually use different instruments that might also be good vectors of plankton dispersal, it is necessary to develop proper uniform biosecurity treatments. No longer facilitating the plankton spread through hydrobiological instruments is the milestone that we, plankton samplers worldwide, should achieve together in the nearest future if we want to continue our desire to explore, understand, protect and save nature.
Keywords
Abiotic resistance, accidental species introduction, aquatic biomonitoring, biosecurity treatment, invasive species
Introduction
Dispersal is one of the main forces that shape the diversity of plankton communities. Numerous plankton species possess various morphological structures (hooks, threads, spines, suckers) and adaptations (buoyancy regulation, resting cells and eggs, gelatinous sheaths) that make them easy to be transported via different vectors for short and long distances. Thanks to dispersal, plankton organisms can invade new habitats, which may lead to establishing a stable population in a new waterbody. Thereby, plankton dispersal has an important influence on the biodiversity of aquatic ecosystems.
Plankton organisms can disperse outside of their existing distributions using different natural vectors: wind, extreme events, channels or rivers, other organisms such as birds, semi-aquatic and land animals (
Because humans will continue to interact with aquatic environments, it is impossible to completely stop the human-aided dispersal of aquatic organisms. What we can do is minimise the risk of occurrence of human-mediated organism dispersal events by the implementation of biosecurity methods in human activity. For example, ballast water is subjected to different disinfection techniques before discharge (
In this study, we checked the possibility of the spread of lake plankton between different water bodies through a plankton net during sampling campaigns and whether disinfection of the plankton net with ethanol after sampling might be an efficient method to prevent the transfer of plankton net hitch-hikers into the next waterbody to be sampled. There were two experiments performed. In the first one, we tested whether plankton sampling with a net could facilitate the spread of some filamentous cyanobacteria and their potential invasion of new water bodies; we also tested if the use of biosecurity would prevent their spreading. Our attention was focused on cyanobacteria – organisms that often constitute a dominant component of plankton in different types of aquatic habitats and form dense blooms, generating an avalanche of problems to the functioning and usage of aquatic ecosystems (
Methods and materials
Dispersal of cyanobacteria via a plankton net, the fate of hitch-hikers, and possible prevention
We checked whether plankton sampling by a net with and without biosecurity could facilitate the spread of cyanobacteria and their invasion of new water bodies. Four laboratory strains of common bloom-forming filamentous cyanobacteria species such as Aphanizomenon gracile, Aphanizomenon flos-aquae, Planktothrix agardhii, and Raphidiopsis raciborskii (Table
Basic information on the origin (lake/reservoir name, GPS coordinates) of examined strains of cyanobacteria and their toxins (symbols +, –, n.e. indicate that a given toxin was detected, not detected or not examined, respectively).
| Toxin | A. flos-aquae | A. gracile | P. agardhii | R. raciborskii | ||||
|---|---|---|---|---|---|---|---|---|
| AMU-DH-6 | AMU-DH-1 | SAG 6.89 | SAG 1.97 | |||||
| Sulejów Reservoir | Lake Buszewskie | Lake Lough Neagh | Lake Balaton | |||||
| 51°26'0"N, 19°55'25"E | 52°32'42"N, 16°22'47"E | 54°11'0"N, 10°26'45"E | 46°48'51"N, 17°45'52"E | |||||
| Anabaenopeptins | – | (†) | + | (‡) | + | (†) | + | (‡) |
| Anatoxin-a | – | (†) | – | (‡) | – | (†) | – | (‡) |
| β-Methylamino-L-alanine | – | (†) | – | (‡) | – | (†) | – | (‡) |
| Cylindrospermopsin | – | (†) | – | (¥) | – | (¶) | – | (¶) |
| Desmethyl microcystin-LR | – | (†) | – | (¥) | + | (¶) | – | (¶) |
| Desmethyl microcystin-RR | – | (†) | – | (¥) | + | (¶) | – | (¶) |
| Microcystin-LR | – | (†) | – | (¥) | + | (§, ¶) | – | (¶) |
| Microcystin-RR | – | (†) | – | (¥) | + | (§, ¶) | – | (¶) |
| Microcystin-YR | – | (†) | – | (¥) | + | (§, ¶) | – | (¶) |
| Microcystin-LF | – | (†) | – | (¥) | – | (¶) | – | (¶) |
| Microcystin-LW | – | (†) | – | (¥) | – | (¶) | – | (¶) |
| Microcystin-LY | – | (†) | – | (¥) | – | (¶) | – | (¶) |
| Nodularin | – | (†) | – | (†) | – | (†) | n.e. | |
| Saxitoxins | – | (†) | – | (‡) | – | (†) | – | (‡) |
Twenty-day-old cyanobacterial cultures were inoculated into 70 L of distilled water. After 5 min. of gently mixing the volume of the container, we collected three subsamples of water for chlorophyll-a and microscopic analyses of cyanobacteria (respectively: 80 ml × 3, 1.5 ml × 3). Subsequently, 10 handmade plankton nets (mesh size: 100 µm; Suppl. material
Dispersal of the wild plankton via a net and the fate of hitch-hikers
Here, we checked whether plankton sampling with a net without biosecurity could facilitate the spread of lake phyto- and zooplankton and their invasions of new water bodies. For this purpose, a shallow postglacial open lake – Gopło (central Poland) was visited and sampled in October 2019. We chose this lake because of its high susceptibility to cyanobacterial blooms known from the past several decades (
At first, we collected three 300 ml subsamples of the lake water for a chlorophyll-a analysis and a 1 L sample for a microscopic analysis. Second, 15 plankton nets (the same nets as used in the first experiment) with open sample buckets were separately and fully immersed for 5 seconds in Gopło’s waters at a depth of ~1.5 m. Subsequently, they were placed freely in plastic bags in a car Volkswagen Caddy III cargo (Volkswagen Group, Wolfsburg, Germany).
After 4.5 hours, the nets were returned to the laboratory. Five randomly selected nets were separately immersed for 5 seconds in beakers filled with 3 L of distilled water. After gently mixing, half of the volume was taken from each beaker and fixed with Lugol’s solution for a qualitative/quantitative phyto- and zooplankton analysis. This procedure was to reveal Gopło’s plankton net hitch-hikers, which infiltrated into a new waterbody. The remaining ten nets were separately immersed for 5 seconds in beakers filled with 3 L of filtrate (GF/C filters, Whatman, Maidstone, United Kingdom) water from another eutrophic lake – Kierskie (western Poland; GPS coordinates: 52°28'25.6"N, 16°46'59.7"E). Five of them were additionally inoculated with 30 ml of a green alga Tetradesmus obliquus (strain SAG 276-3a). This procedure was to see the fate of Gopło’s potential plankton net hitch-hikers in a new waterbody when a resident is present. All beakers were maintained in a walk-in phytotron for ten days.
On day 0 and after ten days, beakers were sampled for a chlorophyll-a analysis (100 ml) and an assessment of P. agardhii biomass (1.5 ml). At the endpoint, we have also collected 50 mL samples for nutrient analyses and 1 L samples for a qualitative/quantitative analysis of the whole phyto- and zooplankton community structure. This procedure was to see the successes and failures of Gopło’s potential plankton net hitch-hikers after ten days in the new water bodies.
Sample analyses
Chlorophyll-a concentration
Samples were filtered through GF/C filters using a glass vacuum filtration kit and analysed spectrophotometrically following grinding of the filters using a mortar and subsequent 24 hours extraction in 90% acetone (
Cyanobacteria and green algae biomasses
Samples (1.5 ml) collected from the experiments were analysed using an inverted microscope Leica DM IL LED (Leica Microsystems, Wetzlar, Germany) equipped with a digital camera Jenoptik ProgRes Speed Xtcore3 (Jenoptik Optical Systems, Jena, Germany). Trichomes of A. gracile, P. agardhii, and R. raciborskii from the first experiment and P. agardhii from the second experiment, were counted in a Sedgewick-Rafter chamber (Cole-Parmer, Vernon Hills, United States of America). Morphometry of cyanobacterial trichomes was assessed using ProgRes image capture software (Jenoptik Optical Systems, Jena, Germany). The thickness (n = 15) and length (n = 30) of randomly selected trichomes of each species were measured in each sample. Then, the average biovolume of each species was calculated using a formula for computing the volume geometric shape corresponding to the geometric shape of a given species (
B(mm3L-1) = Abundance × Biovolume
Phyto- and zooplankton community composition
Samples (1 L) collected from Experiment 2 were subjected to sedimentation for a month, subsequently concentrated up to 50 ml and additionally fixed with Lugol’s solution. The qualitative (the number of taxa and species composition) and quantitative (abundance expressed as the number of individuals of a given taxon/species L-1) analyses of the Gopło’s phytoplankton and Gopło’s hitch-hikers were performed using an Olympus BX 51 microscope with Nomarski differential contrast (Olympus, Tokyo, Japan). Individuals (single cells, coenobia, colonies, filaments with a length of 100 µm) were counted in Fuchs-Rosenthal counting chamber (height: 0.2 mm, area: 0.0625 mm2) at varied magnifications, i.e. microplankton in the whole chamber (512 fields) using 200× and nannoplankton in 256 fields using 400×. Phytoplankton identification was based on current references (e.g.,
The qualitative (the number of taxa and species composition) and quantitative (abundance expressed as the number of individuals of a given taxon/species L-1) analyses of Gopło’s zooplankton hitch-hikers were performed using a light microscope Axioskop 2 MOT+ (Carl Zeiss Light Microscopy, Oberkochen, Germany). Zooplankters of three groups (Cladocera, Copepoda, Rotifera) were determined and counted in a Sedgewick-Rafter chamber. If necessary, some animals were dissected after analysing the content of the whole chamber to be completely certain of the identification. Zooplankton identification followed
Plankton sampling – survey
A short online survey was designed directly to gather information from plankton samplers worldwide about what they do with the plankton net after sampling and what biosecurity treatment they use. Questions in the survey were close-ended and addressed the following issues: frequency of plankton sampling with a net, the number of sampling points a day, the used methods of biosecurity after sampling, profession and degree status of the respondent (Suppl. material
The survey was targeted at samplers who had an activity in the field of water research visible in the form of a scientific publication(s) indexed in the Web of Science citation database (Clarivate Analytics PLC, Philadelphia, United States of America) between the years 2000–2019. We used the keyword “plankton” in the WoS database search box to find proper participants for our survey. The scientists whose e-mails showed up in the database for the above search range were invited to respond to the survey (10,306 potential respondents). The survey was conducted in English and hosted on the SurveyLab online platform (www.surveylab.com; 7 Points Sp. z o.o., Warsaw, Poland). Each participant could complete the survey only once between March 2 and March 15, 2020.
The survey was voluntary and anonymous. Due to the hosting platform, we avoided collecting sensitive data from respondents. Also, we were unable to identify the respondents and link them to their answers. The exception was when a respondent sent an e-mail directly to the handling author of the survey with additional comments, suggestions, and the desire for a broader discussion.
In the analysis of the survey data, we did not include respondents sampling the marine environment. We also excluded from the analysis those respondents that answered to Q3 (“How many water bodies (e.g. lakes, reservoirs, ponds) do/did you sample for plankton using plankton net per day?”) that they sample/sampled always one water body per day while later (Q5: “Do/Did you use different nets for each sampled lake when you sample/sampled more than one on the same day?”) they did not mark the answer “I do/did not sample more than one lake on the same day”. For the same reason, we excluded from the analysis those respondents that marked another answer than “Always one per day” to Q3 while later to Q5, they marked “I do/did not sample more than one lake on the same day”.
Statistical analyses
Statistical analyses and graphs were conducted using the R software version 4.1.2 (
In the case of experiment 2, Yuen’s trimmed means test for two dependent samples was applied to test the difference in chlorophyll-a concentration between the start- and endpoint of the incubation of Gopło’s hitch-hikers without a resident (Resident(–)). This test was used because these data contained one outlier, and data transformation did not solve the problem. The difference in chlorophyll-a concentration between the start- and endpoint of the incubation of Gopło’s hitch-hikers with a resident T. obliquus (Resident(+)) was tested using Welch’s paired t-test. The difference between Resident(–) and Resident(+) treatments in the biomass yield of Gopło’s hitch-hiker P. agardhii at the endpoint of the incubation was tested using Yuen’s trimmed means test. It was due to the presence of one outlier in the Resident(–) treatment.
Data were visualized using GGally (
Results
Dispersal of cyanobacteria via a plankton net, the fate of hitch-hikers, and possible prevention
Cyanobacteria successfully spread into the new environment and withstood there when no biosecurity was applied (Biosecurity(–)), as positive values of the chlorophyll-a concentration and biomass of each species of cyanobacteria were detected immediately after the inoculation (Fig.
Chlorophyll-a concentration and biomass of A. gracile, P. agardhii and R. raciborskii at the start and endpoint of the incubation in Biosecurity(–) and Biosecurity(+) treatments. A dashed line indicates the mean value of a given variable in the container with a simulated bloom of cyanobacteria in which plankton nets were immersed before the experiment.
In the Biosecurity(+) treatment, chlorophyll-a and cyanobacterial trichomes of each species were not detected in samples at both sampling points.
Dispersal of the wild plankton via a net and the fate of hitch-hikers
Chlorophyll-a concentration and phytoplankton
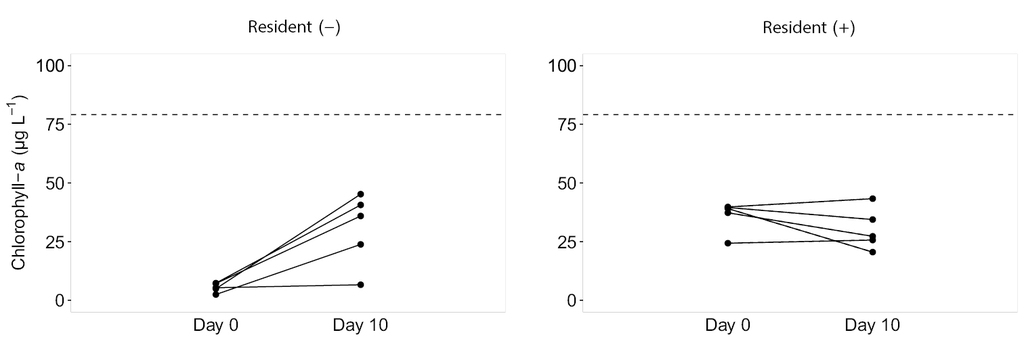
Chlorophyll-a concentration in the Gopło Lake was 79.1 ± 6.89 µg L-1 (mean ± SD). Wild phytoplankton successfully infiltrated the new environment and withstood there regardless of the presence or absence of the resident alga T. obliquus. Chlorophyll-a concentration in beakers without the resident T. obliquus increased over five times during the incubation time (ty = -4.2, DF = 2, p = .052) (Fig.
Gopło’s phytoplankton and hitch-hikers
Phytoplankton in the Gopło lake was represented by eight groups, including Chlorophyta (44 taxa), Bacillariophyceae (31 taxa), Cyanobacteria (10 taxa), Cryptophyceae (7 taxa), Euglenophyceae (5 taxa), Dinophyceae (3 taxa), Chrysophyceae (1 taxon), and Haptophyceae (1 taxon) (Fig.
Phytoplankton community structure in the Gopło lake, phytoplankton hitch-hikers infiltrated into beakers through plankton nets and their fate after ten days of incubation in filtered lake water from the Kierskie lake without and with resident T. obliquus (Resident(–), Resident(+); respectively). Abbreviations: CYANO – Cyanobacteria, CRYPT – Cryptophyceae, BACILL – Bacillariophyceae, CHLOR – Chlorophyta, DINOP – Dinophyceae, HAPTO – Haptophyceae, EUGLE – Euglenophyceae, CHRYS – Chrysophyceae.
Individuals of thirty-three phytoplankton taxa infiltrated the beakers with the Kierskie lake water via plankton nets (Fig.
After ten days of hitch-hikers’ incubation without resident T. obliquus (Resident(–)), thirty-seven phytoplankton taxa were found in beakers (Fig.
After ten days of hitch-hikers’ incubation with resident T. obliquus (Resident(+)), fourteen phytoplankton taxa were found in beakers (Fig.
Effect of resident on Gopło’s dominant hitch-hiker
At the start of the incubation, P. agardhii biomass in Resident(–) and Resident(+) was, respectively, .99 ± .16 and 1.09 ± .19 mm3 L-1 (mean ± SD). After 10 days of incubation, P. agardhii biomass in the Resident(–) treatment increased up to 7.42 ± 3.55 mm3 L-1 and was over a half higher than in the Resident(+) treatment (3.15 ± 1.8 mm3 L-1) (ty = 4.14, DF = 8, p = .01) (Fig.
Gopło’s zooplankton and hitch-hikers
Zooplankton community in Gopło was represented mainly by rotifers (Fig.
Zooplankton community structure in the Gopło lake, zooplankton hitch-hikers infiltrated into beakers through plankton nets and their fates after ten days of the incubation in filtered lake water from the Kierskie lake without and with resident T. obliquus (Resident(–), Resident(+); respectively). Abbreviations: ROTIF – Rotifera, COPEP – Copepoda, CLADO – Cladocera.
Three Rotifer taxa, four Cladocera taxa, one Copepoda taxon, and larval and juvenile forms of Copepoda (Calanoida and Cyclopoida) infiltrated the beakers with the lake water through plankton nets in quantities large enough to be detected upon first sampling (Fig.
Biosecurity measures
After data filtration (see “Plankton sampling – survey” subsection), responses from 388 respondents were used in the analysis of the survey data, including 340 (88%) broadly understood biologists at a university or freelancer biologists, 12 (3%) employees at water quality monitoring service and 36 (9%) people of another profession. The responses came from 58 countries (Suppl. material
Regarding the frequency of plankton sampling, most respondents reported that they sample/have sampled plankton with a plankton net irregularly, including years without sampling (33%). Regular plankton sampling, either usually more than twenty times a year or usually up to ten times a year was reported, respectively, by 21% and 20% of respondents. Regular sampling, usually from eleven to twenty times a year, was reported by 14% of the respondents. Irregular sampling of up to ten times a year in some years and more often in other years was declared by 11% of the respondents. There were also 1% of respondents that marked the response “None of the above”.
Regarding the number of water bodies sampled per day, 39% of respondents declared to sample usually one water body per day and rarely more than one, 38% of the respondents reported to sample usually more than one but not more than five water bodies per day, 17% indicated that they always sample one water body per day. Another 6% of respondents usually sample more than five water bodies per day.
Among those who sample more than one lake a day, only 22.5% of respondents declared to use a different plankton net for each lake, while 77.5% use the same net in all sampled lakes.
A little over a half of the respondents declared to always rinse/have rinsed an open plankton net after sampling with distilled water/tap water and let it dry (Fig.
Biosecurity procedure(s) used by plankton samplers worldwide with the plankton net after sampling. Numbers inside the bars indicate the number of respondents who marked a given response.
The second most popular method used by 19% of the respondents is to always immerse (several times) an open plankton net in the water body and let it dry, while 13% of respondents admitted to always immerse (several times) an open plankton net in the water body. Only 9% of respondents indicated that they always rinse/have rinsed an open plankton net with a detergent or other liquid to disinfect the plankton net. There was also 5% of the respondents who always just leave the plankton net to dry, and 3% of the respondents that marked the “None of the above” response. These last 3% of the respondents were additionally asked to specify what they do with the plankton net after sampling and all the answers are outlined in Table
The detailed explanations that were provided by the 3% of respondents who chose the answer “None of the above” when asked what they do with plankton net after sampling.
| No. | Original answers |
|---|---|
| 1 | “Rinse with distilled water if sampling in the same water body, wash with detergent if using in different water bodies.” |
| 2 | “We use separate plankton nets for each lake - one lake, one plankton net. But we rinse it 10 times after sampling and 10 times before sampling next month.” |
| 3 | “It depends, if there is a risk of spreading invasive species, I sterilise and then dry the net. Otherwise I take it back to the lab and rinse it with distilled water before letting it dry.” |
| 4 | “After sampling I take the plankton net to the next sampling place and rinse there the net several times before sampling.” |
| 5 | “Rinse and preserve in formalin.” |
| 6 | “The sample should be concentrated and the net should be washed and dried.” |
| 7 | “I don´t know it so precisely.” |
| 8 | “Samples after draining are fixed with formalin or alcohol.” |
| 9 | “I added formaldeyhde to a 4% concentration and buffer with hexamethiltetramine until a pH of 8.2.” |
| 10 | “i.” |
Discussion
The results of our experiments demonstrate that a plankton net is an efficient vector for dispersion of plankton organisms. In the first experiment, all examined strains of cyanobacteria were able to disperse from the source into the new medium through the plankton net. They were able to survive three hours on the plankton nets out of the water and inhabit the new medium. There was one dominant species (A. gracile) that increased markedly in its biomass at the endpoint of the incubation; others (P. agardhii, R. raciborskii) maintained a relatively stable biomass during the experiment. There were also visible numerous enlarged A. flos-aquae fascicles at the endpoint of the incubation. All of these species often co-occur and compete for dominance in eutrophic waters of the temperate zone (
Keeping the plankton net in 30% ethanol after sampling seems to be a promising method to prevent the spread of cyanobacteria between different water bodies via the plankton net. After seven days of incubation, neither chlorophyll-a nor cyanobacterial trichomes/fascicles were detected in the medium. This result additionally points to the superiority of the biosecurity method based on disinfection with ethanol over only flushing the net in the water body and letting it dry. The biosecurity method using ethanol does not require a lot of work, time, and space in the vehicle, and is also relatively cheap (it only takes a bucket with a lid + ethanol + distilled/tap water). There are also other possible disinfectant liquids that could be used, but we believed that due to rapid evaporation, ethanol would have negligible negative effects on the environment. The advantage of biosecurity methods based on disinfectant liquids is that their power can be maximized by using a higher concentration of a disinfectant to get an assurance that other planktonic organisms and their propagules will also become irreversibly inactive when transferred into a new water body.
Identifying which phyto- and zooplankton species are effective or ineffective plankton net hitch-hikers was enabled in the second experiment, which was based on plankton from the Gopło Lake. Out of one-hundred and two Gopło’s phytoplankton taxa, more than thirty of them were transferred into beakers with filtered lake water from another lake and survived ten days after the inoculation. A few zooplankton taxa from the Gopło Lake turned out to be successful intruders as well, some of them infiltrated the new simulated water body quite abundantly. There were numerous phytoplankton and only a couple of zooplankton hitch-hikers that expanded their abundance in the lake filtrate within ten days. The higher number of phytoplankton taxa found at the end point of the incubation in comparison to the start means that “no one is excluded from the game” and even those hitch-hikers that were initially easy to overlook due to low abundance can quickly proliferate in the new environment and succeed there. However, organisms with spines, bristles, and other protruding elements can have a greater chance of hitchhiking. For example, rotifer K. cochlearis f. typica with a caudal spine infiltrated the water more effectively than K. cochlearis f. tecta without the spine. This raises the question as to whether such spines provide a better chance of spreading on some of the natural vectors like bird feathers. The dominance of P. agardhii in the Gopło Lake and in the filtrates of the Kierskie Lake at the start- and endpoint of the experiment confirm that bloom-forming cyanobacteria can be easily spread by plankton samplers if there are no efficient biosecurity methods used. Because eutrophic waters are subjected to more frequent biomonitoring (due to e.g. phytoplankton bloom events, ongoing ecosystem restoration) and they often offer good conditions for the growth of nuisance plankton, efficient net hitch-hikers might have consequently a greater chance to spread and expand their distribution range. This might explain to some extent why numerous nuisance plankton taxa are cosmo- or at least subcosmopolitan. In the light of the 1) paradigm “everything is everywhere, but, the environment selects” (
Predicting the fate of hitch-hikers is a great challenge as it depends on the resultant force of environmental factors in the new environment which these organisms and/or their propagules have to face. Among the factors determining winners and losers among newcomers, competitive abilities of native residents and biotic resistance of the whole native communities are often one of the most crucial (
Getting insight into how aquatic ecologists worldwide prevent nowadays the spread of the plankton via the sampling net during their field works was also our “must-know” in this study. As revealed by the survey, less than a tenth of plankton samplers implement a biosecurity treatment based on disinfectant liquids (rinsing the net after sampling with detergent or other disinfectant liquid). The overwhelming majority of the respondents use other ways: 1) rinsing the net with open buckets after sampling with distilled/tap water, 2) immersing the net several times in the water body and letting it dry, 3) immersing the net in the waterbody without taking care about its dryness or 4) just leaving the net to dry. Based on the results of our two experiments, we already know that biosecurity treatment relying on flushing the net with an open bucket after sampling in the waterbody and letting it dry does not prevent plankton spread between the water bodies. This especially refers to the situation when sampling of several water bodies a day is planned, and considering the answers of our respondents on – “How many water bodies (e.g. lakes, reservoirs, ponds) biologists sample using plankton net per day?” – this practice is quite common in multiple sampling cases. Flushing the net after sampling in the water body and letting it dry fails as a biosecurity method also because it does not prevent the spread of plankton propagules (cysts, resting eggs and cells, spores etc.) that can well handle desiccation (
Conclusion
In summary, this study demonstrates that plankton net is an efficient vector for dispersion of plankton organisms. The fate of plankton net hitch-hikers in the new environment is strongly shaped by the native residents. A promising biosecurity method preventing the spread of plankton between water bodies is disinfection of the plankton net with an ethanol solution after sampling. Survey data indicate that the vast majority of people use either ineffective or questionable biosecurity treatments. No longer facilitating the plankton spread is the milestone that we, naturalists worldwide, should achieve together in the nearest future.
Acknowledgements
The authors are grateful to Adrianna Wojtal-Frankiewicz for supplying us with water from Sulejów Reservoir, from which a strain of cyanobacterium was obtained; Sławek Cerbin for supplying us with green algal inoculum; Przemysław Zieliński for technical assistance in the chlorophyll-a analysis. We would also like to thank all anonymous respondents for taking the time to complete our survey.
Erasmus+ student exchange research programme enabled Tümer Orhun Aykut to intern at the Department of Hydrobiology at AMU and participate in the research. The Polish National Agency for Academic Exchange (NAWA) financed a research stay of Łukasz Wejnerowski at Biochemistry, Faculty of Science and Engineering, Åbo Akademi University in Turku (grant no. PPN/BEK/2020/1/00241), during which toxicological analyses were performed.
CRediT authorship contribution statement: Wejnerowski Ł: Conceptualization, Methodology, Software, Validation, Formal analysis, Investigation, Resources, Data Curation, Writing - Original Draft, Writing - Review & Editing, Visualization, Supervision, Project administration. Aykut TO: Methodology, Investigation, Writing - Review & Editing. Pełechata A: Methodology, Software, Investigation, Writing - Review & Editing. Rybak M: Methodology, Software, Investigation, Writing – Review & Editing. Dulić T: Methodology, Investigation, Writing - Review & Editing. Meriluoto J: Methodology, Software, Investigation, Writing - Review & Editing. Dziuba MK: Methodology, Software, Validation, Formal analysis, Investigation, Resources, Data Curation, Writing - Review & Editing.
References
- Amoros C (1984) Introduction Practique à la Systématique des Organismes des Eaux Continentales Françaises – 5. Crustacés Cladocères. Bulletin mensuel de la Société linnéenne de Lyon 53: 72–107. https://doi.org/10.3406/linly.1984.10627
- Antunes JT, Leão PN, Vasconcelos VM (2015) Cylindrospermopsis raciborskii: Review of the distribution, phylogeography, and ecophysiology of a global invasive species. Frontiers in Microbiology 6: e473. https://doi.org/10.3389/fmicb.2015.00473
- Auguie B (2017) gridExtra: Miscellaneous Functions for “Grid” Graphics. R Package Version, 2.3. https://cran.r-project.org/package=gridExtra
- Baas-Becking LGM (1934) Geobiologie of Inleiding Tot de Milieukunde. W.P. Van Stockum & Zoon, The Hague.
- Bass D, Rueckert S, Stern R, Cleary AC, Taylor JD, Ward GM, Huys R (2021) Parasites, pathogens, and other symbionts of copepods. Trends in Parasitology 37(10): 875–889. https://doi.org/10.1016/j.pt.2021.05.006
- Bednarska A, Pietrzak B, Pijanowska J (2014) Effect of poor manageability and low nutritional value of cyanobacteria on Daphnia magna life history performance. Journal of Plankton Research 36(3): 838–847. https://doi.org/10.1093/plankt/fbu009
- Benson A, Maynard E, Raikow D, Larson J, Makled TH, Fusaro A (2022) Daphnia lumholtzi G.O. Sars, 1885: U.S. Geological Survey, Nonindigenous Aquatic Species Database, Gainesville, FL. https://nas.er.usgs.gov/queries/greatlakes/FactSheet.aspx?Species_ID=164
- Bergström AK, Bigler C, Stensdotter U, Lindström ES (2008) Composition and dispersal of riverine and lake phytoplankton communities in connected systems with different water retention times. Freshwater Biology 53(12): 2520–2529. https://doi.org/10.1111/j.1365-2427.2008.02080.x
- Bielańska-Grajner I, Ejsmont-Karabin J, Radwan S (2015) Rotifers – Rotifera Monogononta (first ed.). Łódź University Press & Jagiellonian University Press.
- Błędzki LA, Rybak JI (2016) Freshwater crustacean zooplankton of Europe. Springer. https://doi.org/10.1007/978-3-319-29871-9
- Bøhn T, Amundsen PA, Sparrow A (2008) Competitive exclusion after invasion? Biological Invasions 10(3): 359–368. https://doi.org/10.1007/s10530-007-9135-8
- Buchberger F, Stockenreiter M (2018) Unsuccessful invaders structure a natural freshwater phytoplankton community. Ecosphere 9(3): e02158. https://doi.org/10.1002/ecs2.2158
- Burchardt L, Podolski G (1992) Algological investigations of the Gopło, Pakowskie and South lakes in years 1979–84. In: Bohr R, Nienartowicz A, Wilkoń-Michalska J (Eds) Some ecological processes of the biological systems in North Poland. Nicolas Copernicus University Press, 299–313.
- Carlton JT, Geller JB (1993) Ecological roulette: The global transport of nonindigenous marine organisms. Science 261(5117): 78–82. https://doi.org/10.1126/science.261.5117.78
- Costa OYA, Raaijmakers JM, Kuramae EE (2018) Microbial extracellular polymeric substances: Ecological function and impact on soil aggregation. Frontiers in Microbiology 9: e1636. https://doi.org/10.3389/fmicb.2018.01636
- Cruz D, Vasconcelos V, Pierre G, Michaud P, Delattre C (2020) Exopolysaccharides from cyanobacteria: Strategies for bioprocess development. Applied Sciences (Basel, Switzerland) 10(11): e3763. https://doi.org/10.3390/app10113763
- Dzialowski AR, Lennon JT, Smith VH (2007) Food web structure provides biotic resistance against plankton invasion attempts. Biological Invasions 9(3): 257–267. https://doi.org/10.1007/s10530-006-9030-8
- Ettl H (1983) Chlorophyta I. Phytomonadina. Süßwasserflora von Mitteleuropa, 9. Gustav Fischer Verlag, Stuttgart, New York.
- Ettl H, Gärtner G (1988) Chlorophyta II. Tetrasporales, Chlorococcales, Gloeodendrales. Süßwasserflora von Mitteleuropa, 10. Gustav Fischer Verlag, Jena.
- Falfushynska H, Horyn O, Osypenko I, Rzymski P, Wejnerowski Ł, Dziuba MK, Sokolova IM (2021) Multibiomarker-based assessment of toxicity of central European strains of filamentous cyanobacteria Aphanizomenon gracile and Raphidiopsis raciborskii to zebrafish Danio rerio. Water Research 194: 116923. https://doi.org/10.1016/j.watres.2021.116923
- Frada MJ, Schatz D, Farstey V, Ossolinski JE, Sabanay H, Ben-Dor S, Koren I, Vardi A (2014) Zooplankton may serve as transmission vectors for viruses infecting algal blooms in the ocean. Current Biology 24(21): 2592–2597. https://doi.org/10.1016/j.cub.2014.09.031
- Ger KA, Urrutia-Cordero P, Frost PC, Hansson LA, Sarnelle O, Wilson AE, Lürling M (2016) The interaction between cyanobacteria and zooplankton in a more eutrophic world. Harmful Algae 54: 128–144. https://doi.org/10.1016/j.hal.2015.12.005
- Grossart HP, Dziallas C, Leunert F, Tang KW (2010) Bacteria dispersal by hitchhiking on zooplankton. Proceedings of the National Academy of Sciences of the United States of America 107(26): 11959–11964. https://doi.org/10.1073/pnas.1000668107
- Guillard RRL, Lorenzen CJ (1972) Yellow-green algae with chlorophyllide c. Journal of Phycology 8(1): 10–14. https://doi.org/10.1111/j.1529-8817.1972.tb03995.x
- Havel JE, Hebert PDN (1993) Daphnia lumholtzi in North America: Another exotic zooplankter. Limnology and Oceanography 38(8): 1823–1827. https://doi.org/10.4319/lo.1993.38.8.1823
- Havel JE, Shurin JB (2004) Mechanisms, effects, and scales of dispersal in freshwater zooplankton. Limnology and Oceanography 49(4part2): 1229–1238. https://doi.org/10.4319/lo.2004.49.4_part_2.1229
- Hess-Erga OK, Moreno-Andrés J, Enger Ø, Vadstein O (2019) Microorganisms in ballast water: Disinfection, community dynamics, and implications for management. The Science of the Total Environment 657: 704–716. https://doi.org/10.1016/j.scitotenv.2018.12.004
- Hindák F (1988) Studies on the chlorococcal algae (Chlorophyceae). IV. Biologické Práce XXXIV. Veda SAV, Bratislava.
- Hori K, Okamoto J, Tanji Y, Unno H (2003) Formation, sedimentation and germination properties of Anabaena akinetes. Biochemical Engineering Journal 14(1): 67–73. https://doi.org/10.1016/S1369-703X(02)00136-5
- Hyun B, Shin K, Jang MC, Jang PG, Lee WJ, Park C, Choi KH (2016) Potential invasions of phytoplankton in ship ballast water at South Korean ports. Marine and Freshwater Research 67(12): 1906–1917. https://doi.org/10.1071/MF15170
- Ji X, Verspagen JMH, Stomp M, Huisman J (2017) Competition between cyanobacteria and green algae at low versus elevated CO2: Who will win, and why? Journal of Experimental Botany 68(14): 3815–3828. https://doi.org/10.1093/jxb/erx027
- Kassambara A (2020) rstatix: Pipe-Friendly Framework for Basic Statistical Tests. R package version 0.7.0. https://cran.r-project.org/web/packages/rstatix/index.html
- Kobos J, Błaszczyk A, Hohlfeld N, Toruńska-Sitarz A, Krakowiak A, Hebel A, Sutryk K, Grabowska M, Toporowska M, Kokociński M, Messyasz B, Rybak A, Napiórkowska-Krzebietke A, Nawrocka L, Pełechata A, Budzyńska A, Zagajewski P, Mazur-Marzec H (2013) Cyanobacteria and cyanotoxins in Polish freshwater bodies. Oceanological and Hydrobiological Studies 42(4): 358–378. https://doi.org/10.2478/s13545-013-0093-8
- Kokociński M, Gągała I, Jasser I, Karosienė J, Kasperovičienė J, Kobos J, Koreivienė J, Soininen J, Szczurowska A, Woszczyk M, Mankiewicz-Boczek J (2017) Distribution of invasive Cylindrospermopsis raciborskii in the East-Central Europe is driven by climatic and local environmental variables. FEMS Microbiology Ecology 93(4): fix035. https://doi.org/10.1093/femsec/fix035
- Komárek J (2013) Cyanoprokaryota: 3. Teil/3rd part: Heterocytous genera. In: Büdel B, Gärtner G, Krienitz L, Schagerl L (Eds) Süßwasserflora von Mitteleuropa Bd. 19/3. Springer Spectrum. https://doi.org/10.1007/978-3-8274-2737-3
- Komárek J, Anagnostidis K (1998) Cyanoprocaryota. 1. Teil: Chroococcales. In: Gärtner EH, Heynigh G, Mollenhauer D (Eds) Süßwasserflora von Mitteleuropa 19/1. Gustav Fischer Verlag.
- Komárek J, Anagnostidis K (2005) Cyanoprocaryota. 2. Teil: Oscillatoriales. In: Büdel B, Krienitz L, Gärtner G, Schagerl M (Eds) Süßwasserflora von Mitteleuropa 19/2. Elsevier GmbH.
- Kosiba J, Wilk-Woźniak E, Krztoń W (2019) The effect of potentially toxic cyanobacteria on ciliates (Ciliophora). Hydrobiologia 827(1): 325–335. https://doi.org/10.1007/s10750-018-3783-9
- Krztoń W, Kosiba J, Pociecha A, Wilk-Woźniak E (2019) The effect of cyanobacterial blooms on bio- and functional diversity of zooplankton communities. Biodiversity and Conservation 28(7): 1815–1835. https://doi.org/10.1007/s10531-019-01758-z
- Lange-Bertalot H, Hofmann G, Werum M, Cantonati M (2017) Freshwater benthic diatoms of central Europe: over 800 common species used in ecological assessment. Koeltz Botanical Books.
- Mair P, Wilcox RR (2020) Robust Statistical Methods in R Using the WRS2 Package. Behavior Research Methods 52(2): 464–488. https://doi.org/10.3758/s13428-019-01246-w
- Martín-Torrijos L, Martínez-Ríos M, Casabella-Herrero G, Adams SB, Jackson CR, Diéguez-Uribeondo J (2021) Tracing the origin of the crayfish plague pathogen, Aphanomyces astaci, to the Southeastern United States. Scientific Reports 11(1): e9332. https://doi.org/10.1038/s41598-021-88704-8
- Meccheri FS (2010) Produção e composição dos polissacarídeos extracelulares de Planktothrix agardhii (Cyanobacteria) e suas relações com bactérias no reservatório de Barra Bonita. MSc thesis. Federal University of São Carlos.
- Miglietta MP, Lessios HA (2009) A silent invasion. Biological Invasions 11(4): 825–834. https://doi.org/10.1007/s10530-008-9296-0
- Moustaka-Gouni M, Sommer U (2020) Effects of harmful blooms of large-sized and colonial cyanobacteria on aquatic food webs. Water (Basel) 12(6): e1587. https://doi.org/10.3390/w12061587
- Mur LR, Gons HJ, Van Liere L (1978) Competition of the green alga Scenedesmus and the blue-green alga Oscillatoria. Internationale Vereinigung für Theoretische und Angewandte Limnologie: Mitteilungen 21(1): 473–476. https://doi.org/10.1080/05384680.1978.11903986
- Padisák J (2003) Phytoplankton. In: O’Sullivan PE, Reynolds CS (Eds) The Lakes Handbook: Limnology and Limnetic Ecology. Blackwell Science Ltd. https://doi.org/10.1002/9780470999271.ch10
- Padisák J, Vasas G, Borics G (2016) Phycogeography of freshwater phytoplankton: Traditional knowledge and new molecular tools. Hydrobiologia 764(1): 3–27. https://doi.org/10.1007/s10750-015-2259-4
- Pinero-Rodríguez MJ, Fernández-Zamudio R, Arribas R, Gomez-Mestre I, Díaz-Paniagua C (2020) The invasive aquatic fern Azolla filiculoides negatively impacts water quality, aquatic vegetation and amphibian larvae in Mediterranean environments. Biological Invasions 23(3): 755–769. https://doi.org/10.1007/s10530-020-02402-6
- Popovský J, Pfiester LA (1990) Dinophyceae (Dinoflagellida). Süßwasserflora von Mitteleuropa, 6. Gustav Fischer Verlag, Jena, Stuttgart.
- R Core Team (2021) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. https://www.r-project.org/
- R Studio Team (2022) Rstudio: Integrated Development for R. Rstudio, PBC, Boston, MA. https://www.rstudio.com/
- Radzikowski J (2013) Resistance of dormant stages of planktonic invertebrates to adverse environmental conditions. Journal of Plankton Research 35(4): 707–723. https://doi.org/10.1093/plankt/fbt032
- Ribeiro KF, Duarte L, Crossetti LO (2018) Everything is not everywhere: A tale on the biogeography of cyanobacteria. Hydrobiologia 820(1): 23–48. https://doi.org/10.1007/s10750-018-3669-x
- Rzymski P, Horyn O, Budzyńska A, Jurczak T, Kokociński M, Niedzielski P, Klimaszyk P, Falfushynska H (2018) A report of Cylindrospermopsis raciborskii and other cyanobacteria in the water reservoirs of power plants in Ukraine. Environmental Science and Pollution Research 25(15): 15245–15252. https://doi.org/10.1007/s11356-018-2010-6
- Schloerke B, Cook D, Larmarange J, Briatte F, Marbach M, Thoen E, Elberg A, Crowley J (2021) GGally: Extension to ‘ggplot2’. R package version 2.0.0. https://cran.r-project.org/package=GGally
- Siemińska J (1964) Chrysophyta II. Bacillariophyceae Okrzemki. In: Starmach K (Ed.) Flora słodkowodna Polski, tom 6. PAN, Instytut Botaniki, PWN, Warszawa.
- Sikora AB, Dawidowicz P, Von Elert E (2014) Daphnia fed algal food grown at elevated temperature have reduced fitness. Journal of Limnology 73(3): 421–427. https://doi.org/10.4081/jlimnol.2014.898
- Solarz W, Najberek K, Wilk-Woźniak E, Biedrzycka A (2020) Raccoons foster the spread of freshwater and terrestrial microorganisms–mammals as a source of microbial eDNA. Diversity and Distributions 26(4): 453–459. https://doi.org/10.1111/ddi.13027
- Sorensen KH, Sterner RW (1992) Extreme cyclomorphosis in Daphnia lumholtzi. Freshwater Biology 28(2): 257–262. https://doi.org/10.1111/j.1365-2427.1992.tb00582.x
- Starmach K (1968) Chrysophyta III. Xanthophyceae - Różnowiciowce. In: Starmach K (Ed.) Flora słodkowodna Polski, tom 7. PAN, Instytut Botaniki, PWN, Warszawa-Kraków.
- Starmach K (1989) Plankton roślinny wód słodkich. Metody badania i klucze do oznaczania gatunków występujących w wodach Europy Środkowej. PAN, Zakład Biologii Wód, PWN, Warszawa-Kraków.
- Talling JF (1951) The element of chance in pond populations. The Naturalist 839: 157–170.
- Vadrucci MR, Cabrini M, Basset A (2007) Biovolume determination of phytoplankton guilds in transitional water ecosystems of Mediterranean Ecoregion. Transitional Waters Bulletin 2: 83–102. https://doi.org/10.1285/i1825229Xv1n2p83
- Waajen GWAM, Faassen EJ, Lürling M (2014) Eutrophic urban ponds suffer from cyanobacterial blooms: Dutch examples. Environnmental Science and Polluion Research 21(16): 9983–9994. https://doi.org/10.1007/s11356-014-2948-y
- Weithoff G, Taube A, Bolius S (2017) The invasion success of the cyanobacterium Cylindrospermopsis raciborskii in experimental mesocosms: Genetic identity, grazing loss, competition and biotic resistance. Aquatic Invasions 12(3): 333–341. https://doi.org/10.3391/ai.2017.12.3.07
- Wejnerowski L, Cerbin S, Wojciechowicz M, Jurczak T, Glama M, Meriluoto J, Dziuba M (2018) Effects of Daphnia exudates and sodium octyl sulphates on filament morphology and cell wall thickness of Aphanizomenon gracile (Nostocales), Cylindrospermopsis raciborskii (Nostocales) and Planktothrix agardhii (Oscillatoriales). European Journal of Phycology 53(3): 280–289. https://doi.org/10.1080/09670262.2018.1442585
- Wejnerowski Ł, Falfushynska H, Horyn O, Osypenko I, Kokociński M, Meriluoto J, Jurczak T, Poniedziałek B, Pniewski F, Rzymski P (2020) In vitro toxicological screening of stable and senescing cultures of Aphanizomenon, Planktothrix, and Raphidiopsis. Toxins 12(6): e400. https://doi.org/10.3390/toxins12060400
- Wetzel RG, Likens GE (2000) Limnological Analyses. Springer, 429 pp. https://doi.org/10.1007/978-1-4757-3250-4
- Wickham H (2016) ggplot2: Elegant Graphics for Data Analysis. Springer Verlag, New York. https://doi.org/10.1007/978-0-387-98141-3
- Wilk-Woźniak E, Solarz W, Najberek K, Pociecha A (2016) Alien cyanobacteria: An unsolved part of the “expansion and evolution” jigsaw puzzle? Hydrobiologia 764(1): 65–79. https://doi.org/10.1007/s10750-015-2395-x
- Wołowski K, Hindák F (2003) Atlas of Euglenophytes. VEDA, Publishing House of the Slovak Academy of Sciences, Bratislava.
- Xue X, Lv Y, Liu Q, Zhang X, Zhao Y, Zhang L, Xu S (2015) Extracellular polymeric substance from Aphanizomenon flos-aquae induces apoptosis via the mitochondrial pathway in A431 human epidermoid carcinoma cells. Experimental and Therapeutic Medicine 10(3): 927–932. https://doi.org/10.3892/etm.2015.2644
- Zarantonello V, Silva TP, Noyma NP, Gamalier JP, Mello MM, Marinho MM, Melo RCN (2018) The cyanobacterium Cylindrospermopsis raciborskii (CYRF-01) responds to environmental stresses with increased vesiculation detected at single-cell resolution. Frontiers in Microbiology 9: e272. https://doi.org/10.3389/fmicb.2018.00272
Supplementary materials
Detailed methodology of toxicological analyses performed for the purpose of this study and the results in the graphical form
Data type: text + figures
Explanation note: Detailed methodology of toxicological analyses performed for the purpose of this study and the results in the graphical form. Abbreviations: APs – anabaenopeptins, ATX-a – anatoxin-a, BMAA – β-N-methylamino-L-alanine, CYN – cylindrospermopsin, MCs –microcystins, NOD – nodularin, STXs – saxitoxins.
Scheme of the handmade plankton nets used in the experiments and dimensions
Data type: figure
Explanation note: Scheme of the handmade plankton nets used in the experiments and dimensions.
Basic information about the Gopło Lake and some characteristics of the surface water measured during field campaigns
Data type: Table + text + figures
Explanation note: Basic information about the Gopło Lake and some characteristics of the surface water measured during field campaigns.
The list of questions and possible responses in the survey
Data type: text
Explanation note: The list of questions and possible responses in the survey.
Top view of the content of two randomly selected beakers of Biosecurity(–) treatment after seven days of incubation in a phytotron
Data type: image
Explanation note: Top view of the content of two randomly selected beakers of Biosecurity(–) treatment after seven days of incubation in a phytotron.
Survey responses by country
Data type: figure
Explanation note: Survey responses by country.
Concentration of ammonium, nitrate, orthophosphates in the Kierskie Lake filtrates (Resident(-), Resident(+)) at endpoint of the second experiment
Data type: figure
Explanation note: Concentration of ammonium (NH4+), nitrate (NO3-), orthophosphates (PO43-) in the Kierskie lake filtrates (Resident(-), Resident(+)) at endpoint of the second experiment.