Research Article |
|
Corresponding author: Ivan Špelić ( ispelic@agr.hr ) Academic editor: Ali Serhan Tarkan
© 2022 Ana Marić, Ivan Špelić, Tena Radočaj, Zoran Vidović, Tamara Kanjuh, Lorenzo Vilizzi, Marina Piria, Vera Nikolić, Dubravka Škraba Jurlina, Danilo Mrdak, Predrag Simonović.
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation:
Marić A, Špelić I, Radočaj T, Vidović Z, Kanjuh T, Vilizzi L, Piria M, Nikolić V, Škraba Jurlina D, Mrdak D, Simonović P (2022) Changing climate may mitigate the invasiveness risk of non-native salmonids in the Danube and Adriatic basins of the Balkan Peninsula (south-eastern Europe). In: Giannetto D, Piria M, Tarkan AS, Zięba G (Eds) Recent advancements in the risk screening of freshwater and terrestrial non-native species. NeoBiota 76: 135-161. https://doi.org/10.3897/neobiota.76.82964
|
Abstract
Salmonids are an extensively hatchery-reared group of fishes that have been introduced worldwide mainly for their high commercial and recreational value. The Balkan Peninsula (south-eastern Europe) is characterised by an outstanding salmonid diversity that has become threatened by the introduction of non-native salmonids whose potential risk of invasiveness in the region remains unknown and especially so under predicted climate change conditions. In this study, 13 extant and four horizon non-native salmonid species were screened for their risk of invasiveness in the Danube and Adriatic basins of four Balkan countries. Overall, six (35%) of the screened species were ranked as carrying a high risk of invasiveness under current climate conditions, whereas under predicted conditions of global warming, this number decreased to three (17%). Under current climate conditions, the very high risk (‘top invasive’) species were rainbow trout Oncorhynchus mykiss and brown trout Salmo trutta (sensu stricto), whereas under predicted climate change, this was true only of O. mykiss. A high risk was also attributed to horizon vendace Coregonus albula and lake charr Salvelinus namaycush, and to extant Atlantic salmon Salmo salar and brook trout Salvelinus fontinalis, whose risk of invasiveness, except for S. fontinalis, decreased to medium. For the other eleven medium-risk species, the risk score decreased under predicted climate change, but still remained medium. The outcomes of this study reveal that global warming will influence salmonids and that only species with wider temperature tolerance, such as O. mykiss will likely prevail. It is anticipated that the present results may contribute to the implementation of appropriate management plans to prevent the introduction and translocation of non-native salmonids across the Balkan Peninsula. Additionally, adequate measures should be developed for aquaculture facilities to prevent escapees of non-native salmonids with a high risk of invasiveness, especially into recipient areas of high conservation value.
Keywords
AS-ISK, extant, fish, horizon, invasive, risk screening
Introduction
Following the exponential increase in recent years in the number of introduced species worldwide (
Amongst freshwater fishes, salmonids are one of the most widely introduced groups (
Located in south-eastern Europe, the Balkan Peninsula was a glacial refugium for a large number of endemic species (
The exact period of first introduction, re-introduction and translocation of salmonids in the Balkan Peninsula remains unknown, though in the past century these activities have intensified considerably as a result of re-stocking for recreational fishing (
Previous risk screenings have been carried out for salmonid species partly covering the Danube and Adriatic basins of the Balkan Peninsula (
To fill the above knowledge gap, the aims of this study were to: (i) identify the translocated and introduced salmonid species of the Danube and Adriatic basins of the Balkan Peninsula; (ii) identify by horizon scanning which non-native salmonid species might enter the Balkan Peninsula in the (near) future from neighbouring countries; and (iii) evaluate the risk of invasiveness of both the identified extant and horizon salmonids under current and future (predicted) climate conditions for the risk assessment area. Given their extensive use in aquaculture, regular monitoring of the invasiveness of non-native salmonids is crucial to achieve better management of the native freshwater biota of the Balkan Peninsula with the aim of improving appropriate conservation measures.
Methods
Risk assessment area
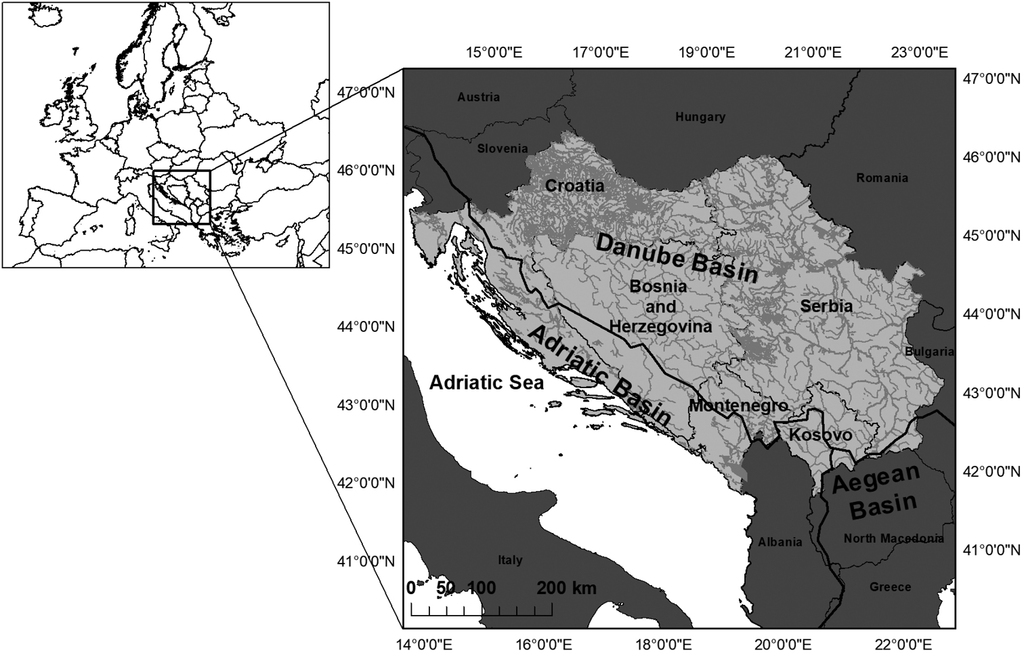
The risk assessment area includes the Danube and Adriatic basins of Bosnia and Herzegovina, Croatia, Montenegro and Serbia (including Kosovo) (Fig.
Map of the risk assessment area (Danube and Adriatic Basins of Bosnia and Herzegovina, Croatia, Montenegro and Serbia with Kosovo) and neighbouring countries for evaluating the potential invasiveness of non-native salmonids.
The Danube Basin includes large lowland rivers, amongst which the most important, besides the River Danube, are the River Sava (Bosnia and Herzegovina, Croatia, Serbia) and the River Tisa (Serbia). The largest river of the Adriatic Basin is the River Neretva (Bosnia and Herzegovina, Croatia). Several other large rivers are present, though the main characteristic of the Adriatic Basin’s hydrology is the presence of numerous karst-sinking rivers, springs and perennial streams (
The Balkan Peninsula is characterised by a remarkable diversity of native salmonids, especially in the countries of Bosnia and Herzegovina (
Species selection
In total, 17 salmonid species were included as part of the risk screening (Table
- Native species translocated from the Danube Basin to the Adriatic Basin (n = 2: Thymallus thymallus and Salmo labrax, which also includes the tentative Salmo taleri);
- Native species translocated outside their native range, but within the Danube Basin (n = 1: Hucho hucho);
- Native species translocated outside their native range, but within the Adriatic Basin (n = 1: Salmo obtusirostris);
- Native species translocated from the Aegean Basin to the Danube Basin (n = 1: Salmo macedonicus).
- Non-native species already present and naturalised/acclimatised in one or more drainage basins (n = 8: European whitefish Coregonus lavaretus, peled Coregonus peled, Oncorhynchus mykiss, Ohrid trout Salmo letnica, Salmo salar, Salmo trutta (sensu stricto), Arctic charr Salvelinus alpinus, brook trout Salvelinus fontinalis);
- Horizon species, i.e. not yet reported, but likely to enter the risk assessment area in the near future (n = 4: lake trout
Salvelins namaycush, lake charr
Salvelinus umbla, Chinook salmon
Oncorhynchus tshawytscha, vendace
Coregonus albula). These species were selected by using the CABI scanning tool (www.cabi.org/horizonscanningtool) for each country in the risk assessment area separately and by literature searches (e.g.
Ventura et al. 2017 ;Radočaj et al. 2021 ), including studies in the native language and ‘grey’ literature.
Extant and horizon non-native salmonids evaluated for their potential risk of invasiveness in the Danube and Adriatic Basins of Bosnia and Herzegovina, Croatia, Montenegro and Serbia (including Kosovo) – the risk assessment area. The criteria for selection of species are: 1 = Native species translocated from the Danube Basin to the Adriatic Basin; 2 = Native species translocated outside their native range but within the Danube Basin; 3 = Native species translocated outside their native range, but within the Adriatic Basin; 4 = Native species translocated from the Aegean Basin to the Danube Basin; 5 = Non-native species already present and naturalised/acclimatised in one or more drainage basins; 6 = Horizon species, i.e. not yet reported but likely to enter the risk assessment area in the near future. For extant species, details about the native distribution area are provided including the location and year of introduction. For all species, the a priori categorisation outcome into Non-invasive and Invasive is provided, based on a multi-tiered protocol (after
| Taxon name | Common name | Criterion | Distribution area | A priori categorisation | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Native | Introduced | Year | FishBase | GISD | CABI | IESNA | GScholar | Outcome | |||
| Extant | |||||||||||
| Coregonus lavaretus | European whitefish | 5 | Northern Europe | Plitvice lakes, Peruča Reservoir, River Cetina | 1937 | N | – | – | – | N | Non-invasive |
| Coregonus peled | peled | 5 | Northern Europe | Plitvice Lakes, Peruča reservoir, River Cetina | 1937 | – | – | – | – | N | Non-invasive |
| Hucho hucho | huchen | 2 | Europe | Rivers Đetinja, Jerma, Nišava, Mlava, Moravica | 2001 | N | – | – | – | N | Non-invasive |
| Oncorhynchus mykiss | rainbow trout | 5 | North America | Vlasina Reservoir | 1792 | Y | Y | Y | Y | n.a. | Invasive |
| Salmo labrax | Black Sea salmon | 1 | Eurasia | Rivers Gacka, Vrijeka | 1948 | N | – | – | – | N | Non-invasive |
| Salmo letnica | Ohrid trout | 5 | Europe, Lake Ohrid | Vlasina Reservoir | 1950 | N | n.e. | – | – | N | Invasive |
| Salmo macedonicus | Macedonian trout | 4 | Central Europe | River Jerma | 2000 | N | – | – | – | N | Non-invasive |
| Salmo obtusirostris | soft-muzzled trout | 3 | Europe, Adriatic Basin | River Žrnovnica | 1970s | N | – | – | – | N | Non-invasive |
| Salmo salar | Atlantic salmon | 5,6 | Northern Europe | Krka Estuary, rivers Sava and Drava | 1980 | N | N | Y | – | n.a. | Invasive |
| Salmo trutta (sensu stricto) | brown trout | 5 | Western Europe | Rivers Gacka, Gradac, Vratna | 1970 | Y | Y | Y | Y | n.a. | Invasive |
| Salvelinus alpinus | Arctic charr | 5 | Northern Europe | Plitvice lakes, River Neretva, Peruča accumulation, Lake Kokin Brod | 1963 | N | – | – | – | N | Non-invasive |
| Salvelinus fontinalis | brook trout | 5 | North America | Plitvice lakes, River Neretva, Peruča accumulation, Lake Kokin Brod | 1960 | Y | Y | Y | – | n.a. | Invasive |
| Thymallus thymallus | grayling | 1 | Eastern Europe | Rivers Cetina, Gacka, Istria, Neretva, Rude | 1960 | N | – | – | – | N | Non-invasive |
| Horizon | |||||||||||
| Coregonus albula | vendace | 6 | – | – | – | N | Y | – | – | n.a. | Invasive |
| Oncorhynchus tshawytscha | chinook salmon | 5 | – | – | – | – | N | – | – | N | Non-invasive |
| Salvelinus namaycush | lake charr | 6 | – | – | – | N | Y | Y | – | n.a. | Invasive |
| Salvelinus umbla | Alpine charr | 6 | – | – | – | N | – | – | – | N | Non-invasive |
Risk screening
Risk screening was undertaken using the Aquatic Species Invasiveness Screening Kit (AS-ISK:
To achieve a valid screening, the assessor must provide for each question a response, a level of confidence for the response (see below) and a justification based on literature sources. The outcomes are a BRA score and a (composite) BRA+CCA score, which is obtained after adding or subtracting up to 12 points to the BRA score or leaving it unchanged in case of a CCA score equal to 0. Scores < 1 suggest that the species poses a ‘low risk’ to become invasive in the risk assessment area, whereas scores ≥ 1 indicate a ‘medium risk’ or a ‘high risk’. The threshold (Thr) value to distinguish between medium-risk (BRA and BRA+CCA score < Thr) and high-risk (BRA and BRA+CCA score ≥ Thr) species for the risk assessment area is obtained by ‘calibration’ based on the Receiver Operating Characteristic (ROC) curve analysis (see
For the ROC curve analysis to be implemented, the species selected for screening must be categorised a priori as ‘non-invasive’ or ‘invasive’ using literature sources. The a priori categorisation of species was implemented as per
CF = ∑(CLQi)/(4 × 55) (i = 1, …, 55)
where CLQi is the CL for Qi, 4 is the maximum achievable value for confidence (i.e. very high: see above) and 55 is the total number of questions comprising the AS-ISK questionnaire (
Implementation of the ROC curve analysis followed the protocol described in
Results
Across all four assessors (Fig.
Box-and-whisker plots showing the Aquatic Species Invasiveness Screening Kit (AS-ISK) outcome scores (Basic Risk Assessment, BRA: light grey; BRA + Climate Change Assessment, BRA+CCA: dark grey) for the four assessors (AM = Ana Marić; IŠ = Ivan Špelić; TK = Tamara Kanjuh; TR = Tena Radočaj) screening the non-native salmonids for the risk assessment area (see Fig.
There were differences in AUCs between AM and TK (P < 0.01), whose AUC had a much lower value (i.e. 0.6143, hence below acceptable discriminatory power) compared to the AUCs from AM, IŠ and TR (i.e. 0.9143, 0.8000 and 0.8786, respectively, hence with excellent to outstanding discriminatory power). As a result, the BRA score outcomes from TK were removed from subsequent analyses and the threshold value was computed, based on the mean BRA scores from AM, IŠ and TR. The ROC curve resulted in an AUC of 0.9286 (0.7810–1.0000 95% CI), which indicated outstanding discriminatory power. Youden’s J provided the threshold of 19.25, which was used for calibration of the risk outcomes. Accordingly, based on the BRA scores, the threshold allowed the distinction of medium-risk species with scores within the interval [1, 19.25 [from high-risk species with scores within [19.25, 68]; based on the BRA+CCA scores, the threshold allowed the distinction of medium-risk species with scores within the interval [1, 19.25 [from high-risk species with scores within [19.25, 80]. Low-risk species had BRA scores within [−20, 1 [and BRA+CCA scores within [−32, 1 [(see Table
- Based on the BRA outcome scores (Table
3 ): six (35.3%) species were classified as high risk and eleven (64.7%) as medium risk. Amongst the seven species categorised a priori as invasive, six were true positives (Coregonus albula, Oncorhynchus mykiss, Salmo salar, Salmo trutta, Salvelinus fontinalis, Salvelinus namaycush). Of the eleven medium-risk species, ten were a priori non-invasive and one invasive. - Based on the BRA+CCA outcome scores, hence after accounting for climate change predictions (Table
3 ): three (17.6%) species were classified as high risk, 13 (76.5%) as medium risk and one (5.9%) as low risk (Hucho hucho). Amongst the a priori invasive species, three were true positives (Oncorhynchus mykiss, Salmo trutta, Salvelinus fontinalis) and, amongst the ten species categorised a priori as non-invasive, one was a truer negative (Hucho hucho). Of the 13 medium-risk species, nine were a priori non-invasive and four invasive.
Permutational ANOVA results for the Aquatic Species Invasiveness Screening Kit (AS-ISK) outcome scores and for the confidence factor (CF) of the non-native salmonids screened for the risk assessment area. Component = BRA, BRA+CCA (see Table
| Source of variation | df | MS | F #/t | P# |
|---|---|---|---|---|
| Scores | ||||
| Component | 1 | 6.431 | 7.177 | 0.009 |
| Assessor | 3 | 4.578 | 5.109 | 0.002 |
| AM vs. IŠ | 1 | – | 3.402 | < 0.001 |
| AM vs. TK | 1 | – | 3.228 | 0.003 |
| AM vs. TR | 1 | – | 2.522 | 0.014 |
| IŠ vs. TK | 1 | – | 0.343 | 0.734 |
| IŠ vs. TR | 1 | – | 0.717 | 0.476 |
| TK vs. TR | 1 | – | 0.928 | 0.352 |
| Component × Assessor | 3 | 0.045 | 0.050 | 0.984 |
| Residual | 128 | 0.896 | ||
| CF | ||||
| Component | 1 | 10.540 | 24.515 | < 0.001 |
| Assessor | 2 | 23.664 | 55.040 | < 0.001 |
| AM vs. IŠ | 1 | – | 5.058 | < 0.001 |
| AM vs. TR | 1 | – | 10.111 | < 0.001 |
| IŠ vs. TR | 1 | – | 5.604 | < 0.001 |
| Component × Assessor | 2 | 0.929 | 2.162 | 0.123 |
| Residual | 96 | 0.430 | ||
The highest-scoring species (BRA and BRA+CCA scores > 30, taken as an ad hoc ‘very high risk’ threshold) were Oncorhynchus mykiss and Salmo trutta for both the BRA and BRA+CCA and Oncorhynchus mykiss only for the CCA. The CCA resulted in a slight increase in the BRA score for only one species (Oncorhynchus mykiss), in no change for another species (Salmo macedonicus) and in a decrease for the remaining 15 species (Table
The mean CFTotal was 0.707 ± 0.017 SE, the mean CFBRA 0.720 ± 0.018 and the mean CFCCA 0.593 ± 0.020. Across the three assessors (i.e. AM, IŠ and TR), the mean CFBRA was significantly higher than the mean CFCCA and the overall CF (i.e. for the BRA and CCA) for assessor AM (0.792 ± 0.112) was significantly higher than that for assessors IŠ (0.663 ± 0.135) and TR (0.515 ± 0.147), which also differed significantly. However, there was no interaction term, indicating that CFBRA and CFCCA did not differ between each other depending on the assessor (Table
Risk outcomes for the non-native salmonids screened with AS-ISK for the risk assessment area. For each species, the following information is provided: a priori categorisation of invasiveness (N = non-invasive; Y = invasive: see Table
| Taxon name | A priori | BRA | BRA+CCA | Delta | CF | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Score | Outcome | Class | Score | Outcome | Class | Total | BRA | CCA | |||
| Coregonus albula | Y | 19.3 | H | TP | 10.0 | M | – | −9.3 | 0.64 | 0.64 | 0.58 |
| Coregonus lavaretus | N | 18.7 | M | – | 14.0 | M | – | −4.7 | 0.74 | 0.77 | 0.54 |
| Coregonus peled | N | 14.5 | M | – | 10.5 | M | – | −4.0 | 0.73 | 0.75 | 0.57 |
| Hucho hucho | N | 10.0 | M | – | 0.0 | L | TN | −10.0 | 0.75 | 0.76 | 0.61 |
| Oncorhynchus mykiss | Y | 33.7 | VH | TP | 42.3 | VH | TP | 8.7 | 0.86 | 0.88 | 0.72 |
| Oncorhynchus tshawytscha | N | 17.5 | M | – | 13.5 | M | – | −4.0 | 0.70 | 0.70 | 0.68 |
| Salmo labrax | N | 19.2 | M | – | 15.2 | M | – | −4.0 | 0.70 | 0.71 | 0.64 |
| Salmo letnica | Y | 15.8 | M | – | 11.2 | M | – | −4.7 | 0.64 | 0.65 | 0.60 |
| Salmo macedonicus | N | 18.3 | M | – | 17.0 | M | – | −1.3 | 0.57 | 0.58 | 0.44 |
| Salmo obtusirostris | N | 8.0 | M | – | 2.0 | M | – | −6.0 | 0.72 | 0.72 | 0.75 |
| Salmo salar | Y | 22.2 | H | TP | 17.5 | M | – | −4.7 | 0.65 | 0.68 | 0.44 |
| Salmo trutta | Y | 32.8 | VH | TP | 26.8 | H | TP | −6.0 | 0.76 | 0.78 | 0.58 |
| Salvelinus alpinus | N | 19.2 | M | – | 13.2 | M | – | −6.0 | 0.72 | 0.73 | 0.61 |
| Salvelinus fontinalis | Y | 29.8 | H | TP | 24.5 | H | TP | −5.3 | 0.76 | 0.79 | 0.53 |
| Salvelinus namaycush | Y | 24.5 | H | TP | 15.8 | M | – | −8.7 | 0.66 | 0.67 | 0.57 |
| Salvelinus umbla | N | 9.8 | M | – | 3.8 | M | – | −6.0 | 0.63 | 0.63 | 0.63 |
| Thymallus thymallus | N | 14.8 | M | – | 8.8 | M | – | −6.0 | 0.80 | 0.82 | 0.58 |
Discussion
Risk outcomes
In this study, the risk of invasiveness of 17 salmonids was determined with a very high level of accuracy (cf. discriminatory power), based on independent assessors. According to the threshold value of 19.25, based on the BRA, only six (35%) species were classified as carrying a high risk of invasiveness for the risk assessment area, whereas based on the BRA+CCA, this number decreased to three (17%). A similar decrease in score for salmonids under predicted climate change scenarios has been observed for Croatia and Slovenia (
Of the screened species, seven were found to pose a high to very high risk of invasiveness for the RA area under current climate conditions (BRA). However, after accounting for predicted climate change conditions (CCA), for four of these species, the risk of invasiveness decreased from high to medium (Table
Oncorhynchus mykiss is a top predator whose negative effects in its introduced range resulting from its carnivorous diet have been documented worldwide (
Salmo trutta (sensu stricto) is one of the most attractive recreational salmonids in the risk assessment area that, however, poses a major threat to the native salmonids because of genetic contamination. Introgression of alien Atlantic haplotypes into the indigenous Salmo labrax and Salmo obtusirostris gene pool has already been documented (
Salvelinus fontinalis is a valuable species for angling both in the risk assessment area and worldwide (
The three a priori invasive species Coregonus albula, Salmo salar and Salvelinus namaycush gained a high risk of invasiveness under current climate conditions (cf. BRA) whereas under the BRA+CCA, their risk became medium. Coregonus albula and Salmo salar are characterised by behavioural and developmental plasticity, which makes them capable to react and potentially adapt to variation in environmental conditions. However, there are limitations to these capacities, especially over short periods of time (
Salmo letnica was the only a priori invasive species found to carry a medium risk of invasiveness likely due to its low dispersal mechanism traits, but also to the scarce data available to answer the AS-ISK questions about ‘undesirable traits’ (see
Climate change
As cold-water species, salmonids are likely to be strongly affected by climate change. An increase in temperature and a decrease in precipitation can directly influence water levels in rivers and lakes (e.g.
Implications for aquaculture
The most suitable streams for salmonid farming in the risk assessment area are in Montenegro, western Croatia and Bosnia-Herzegovina because of the presence of extensive areas with higher altitudes and boreal climate conditions. Interestingly, all salmonid farming in the risk assessment area and surrounding countries (i.e. Albania, Bulgaria, North Macedonia) is based on non-native species with Oncorhynchus mykiss being predominant (
Overall, it is advised that non-native species introductions should be brought to a minimum or avoided altogether and that every introduction of a new species should be conducted only after a full risk assessment (e.g.
Management actions
In the countries of the risk assessment area, freshwater fishing is regulated by different fisheries acts. For example, in Serbia, stocking is limited by law to native species only (
Possibly the most challenging (and still unrecognised) problem for the Balkan Peninsula is the legal stocking of salmonid streams with Salmo trutta (sensu stricto), which poses a threat to native genetic integrity (
Control and containment of introduced salmonids, once established, is the only advisable approach, since eradication is virtually impossible in river systems and large lakes (
Acknowledgements
Special thanks to Linda Zanella for providing constructive comments on an earlier draft of the manuscript. This research was supported by the Croatian Science Foundation (grant IP-2016-06-2563 “Climate change and invasive species – assessing effects on the biodiversity of native freshwater crayfish and salmonids and their conservation”), by the Croatia-Serbia bilateral programme 2019–2021 and by the Ministry of Education, Science and Technological Development of the Republic of Serbia (grant 451-03-9/2021-14/200178).
References
- Andreji J, Stráňai I (2013) Growth parameters of huchen Hucho hucho (L.) in the wild and under culture conditions. Fisheries & Aquatic Life 21: 179–188. https://doi.org/10.2478/aopf-2013-0015
- Araki H, Schmid C (2010) Is hatchery stocking a help or harm? Evidence, limitations, and future directions in ecological and genetic surveys. Aquaculture 308: S2–S11. https://doi.org/10.1016/j.aquaculture.2010.05.036
- Bănărescu PM (2004) Distribution pattern of the aquatic fauna of the Balkan Peninsula. In: Griffiths HI, Kryštufek B, Reed JM (Eds) Balkan biodiversity – Pattern and process in the European hotspot. Springer Science Business Media, Dordrecht, 203–218. https://doi.org/10.1007/978-1-4020-2854-0_12
- Benjamin JR, Connolly PJ, Romine JG, Perry RW (2013) Potential effects of changes in temperature and food resources on life history trajectories of juvenile Oncorhynchus mykiss. Transactions of the American Fisheries Society 142(1): 208–220. https://doi.org/10.1080/00028487.2012.728162
- Bernatchez L (2001) The evolutionary history of brown trout (Salmo trutta L.) inferred from phylogeographic, nested clade, and mismatch analyses of mitochondrial DNA variation. Evolution; International Journal of Organic Evolution 55(2): 351–379. https://doi.org/10.1111/j.0014-3820.2001.tb01300.x
- Berrebi P, Jesenšek D, Laporte M, Crivelli AJ (2022) Restoring marble trout genes in the Soča River (Slovenia). Conservation Genetics. https://doi.org/10.1007/s10592-022-01430-0
- Bilge G, Filiz H, Yapici S, Tarkan AS, Vilizzi L (2019) A risk screening study on the potential invasiveness of Lessepsian fishes in the south-western coasts of Anatolia. Acta Ichthyologica et Piscatoria 49(1): 23–31. https://doi.org/10.3750/AIEP/02422
- Boer P, Loss AC, Bakker F, Beentjes K, Fisher B (2020) Monomorium sahlbergi Emery, 1898 (Formicidae, Hymenoptera): A cryptic globally introduced species. ZooKeys 979: 87–97. https://doi.org/10.3897/zookeys.979.55342
- Britton JR, Gozlan RE, Copp GH (2011) Managing non-native fish in the environment. Fish and Fisheries 12(3): 256–274. https://doi.org/10.1111/j.1467-2979.2010.00390.x
- Buj I, Raguž L, Marčić Z, Ćaleta M, Duplić A, Zanella D, Mustafić P, Ivić L, Horvatić S, Karlović R (2021) Plitvice Lakes National park harbors ancient, yet endangered diversity of trout (genus Salmo). Journal of Applied Ichthyology 37(1): 20–37. https://doi.org/10.1111/jai.14120
- Buoro M, Olden JD, Cucherousset J (2016) Global Salmonidae introductions reveal stronger ecological effects of changing intraspecific compared to interspecific diversity. Ecology Letters 19(11): 1363–1371. https://doi.org/10.1111/ele.12673
- Butchart SHM, Walpole M, Collen B, Van Strien A, Scharlemann JPW, Almond REA, Baillie JEM, Bomhard B, Brown C, Bruno J, Carpenter KE, Carr GM, Chanson J, Chenery AM, Csirke J, Davidson NC, Dentener F, Foster M, Galli A, Galloway JN, Genovesi P, Gregory RD, Hockings M, Kapos V, Lamarque J-F, Leverington F, Loh J, McGeoch MA, McRae L, Minasyan A, Hernández Morcillo M, Oldfield TEE, Pauly D, Quader S, Revenga C, Sauer JR, Skolnik B, Spear D, Stanwell-Smith D, Stuart SN, Symes A, Tierney M, Tyrrell TD, Vié J-C, Watson R (2010) Global biodiversity: Indicators of recent declines. Science 328(5982): 1164–1168. https://doi.org/10.1126/science.1187512
- CABI (2021) Invasive species compendium. CAB International, Wallingford. www.cabi.org/isc
- Cadwallader PL (1996) Overview of the impacts of introduced salmonids on Australian native fauna. Australian Nature Conservation Agency, Canberra, 64 pp.
- Čanak Atlagić J, Marić A, Tubić B, Andjus S, Đuknić J, Marković V, Paunović M, Simonović P (2021) What’s on the menu for the resident brown trout in a rich limestone stream? Water (Basel) 13(18): e2492. https://doi.org/10.3390/w13182492
- Cerri J, Ciappelli A, Lenuzza A, Zaccaroni M, Nocita A (2018) Recreational angling as a vector of freshwater invasions in Central Italy: Perceptions and prevalence of illegal fish restocking. Knowledge and Management of Aquatic Ecosystems 419(419): e38. https://doi.org/10.1051/kmae/2018028
- Cochrane K, De Young C, Soto D, Bahri T (2009) Climate change implications for fisheries and aquaculture. FAO Fisheries and Aquaculture Technical Paper 530: 1–212. http://www.lis.edu.es/uploads/07483fb7_72a2_45ca_b8e7_48bf74072fd3.pdf
- Copp GH, Vilizzi L, Tidbury H, Stebbing PD, Tarkan AS, Miossec L, Goulletquer P (2016) Development of a generic decision-support tool for identifying potentially invasive aquatic taxa: AS-ISK. Management of Biological Invasions 7(4): 343–350. https://doi.org/10.3391/mbi.2016.7.4.04
- Copp GH, Vilizzi L, Wei H, Li S, Piria M, Al-Faisal AJ, Almeida D, Atique U, Al-Wazzan Z, Bakiu R, Bašić T, Bui TD, Canning-Clode J, Castro N, Chaichana R, Çoker T, Dashinov D, Ekmekçi FG, Erős T, Ferincz Á, Ferreira T, Giannetto D, Gilles Jr AS, Głowacki Ł, Goulletquer P, Interesova E, Iqbal S, Jakubčinová K, Kanongdate K, Kim JE, Kopecký O, Kostov V, Koutsikos N, Kozic S, Kristan P, Kurita Y, Lee HG, Leuven RSEW, Lipinskaya T, Lukas J, Marchini A, González-Martínez AI, Masson L, Memedemin D, Moghaddas SD, Monteiro J, Mumladze L, Naddafi R, Năvodaru I, Olsson KH, Onikura N, Paganelli D, Pavia Jr RT, Perdikaris C, Pickholtz R, Pietraszewski D, Povž M, Preda C, Ristovska M, Rosíková K, Santos JM, Semenchenko V, Senanan W, Simonović P, Smeti E, Števove B, Švolíková K, Ta KAT, Tarkan AS, Top N, Tricarico E, Uzunova E, Vardakas L, Verreycken H, Zięba G, Mendoza R (2021) Speaking their language – Development of a multilingual decision-support tool for communicating invasive species risks to decision makers and stakeholders. Environmental Modelling & Software 135: e104900. https://doi.org/10.1016/j.envsoft.2020.104900
- Crisp DT (2000) Trout and salmon: Ecology, conservation and rehabilitation. Blackwell Science, 212 pp. https://doi.org/10.1002/9780470999776
- Crowl TA, Townsend CR, Mcintosh AR (1992) The impact of introduced brown and rainbow trout on native fish: The case of Australasia. Reviews in Fish Biology and Fisheries 2(3): 217–241. https://doi.org/10.1007/BF00045038
- Crozier LG, Hendry AP, Lawson PW, Quinn TP, Mantua NJ, Battin J, Shaw RG, Huey RB (2008) Potential responses to climate change in organisms with complex life histories: Evolution and plasticity in Pacific salmon. Evolutionary Applications 1(2): 252–270. https://doi.org/10.1111/j.1752-4571.2008.00033.x
- Cucherousset J, Aymes JC, Santoul F, Céréghino R (2007) Stable isotope evidence of trophic interactions between introduced brook trout Salvelinus fontinalis and native brown trout Salmo trutta in a mountain stream of south-west France. Journal of Fish Biology 71: 210–223. https://doi.org/10.1111/j.1095-8649.2007.01675.x
- Cucherousset J, Aymes JC, Poulet N, Santoul F, Céréghino R (2008) Do native brown trout and non-native brook trout interact reproductively? Naturwissenschaften 95(7): 647–654. https://doi.org/10.1007/s00114-008-0370-3
- De Silva SS (2012) Aquaculture—A newly emergent food production sector- and perspectives of its impacts on biodiversity and conservation. Biodiversity and Conservation 21(12): 3187–3220. https://doi.org/10.1007/s10531-012-0360-9
- Ficke AD, Myrick CA, Hansen LJ (2007) Potential impacts of global climate change on freshwater fisheries. Reviews in Fish Biology and Fisheries 17(4): 581–613. https://doi.org/10.1007/s11160-007-9059-5
- Franke I (1913) Colourful trout (Trutta iridea) in red trout (Salmo fontinalis). Lovec 4: 130–131. [in Croatian]
- García-Berthou E, Alcaraz C, Pou-Rovira Q, Zamora L, Coenders G, Feo C (2005) Introduction pathways and establishments rates of invasive aquatic species in Europe. Canadian Journal of Fisheries and Aquatic Sciences 62(2): 453–463. https://doi.org/10.1139/f05-017
- Garcia de Leaniz C, Fleming IA, Einum S, Verspoor E, Jordan WC, Consuegra S, Aubin-Horth N, Lajus D, Letcher BH, Youngson AF, Webb JH, Vøllestad LA, Villanueva B, Ferguson A, Quinn TP (2007) A critical review of adaptive genetic variation in Atlantic salmon: Implications for conservation. Biological Reviews of the Cambridge Philosophical Society 82(2): 173–211. https://doi.org/10.1111/j.1469-185X.2006.00004.x
- Gesundheit P, Macias Garcia C (2018) The role of introduced species in the decline of a highly endemic fish fauna in Central Mexico. Aquatic Conservation 6(6): 1384–1395. https://doi.org/10.1002/aqc.2927
- GISD (2021) The Invasive Species Specialist Group ISSG – Global Invasive Species Database. http://www.issg.org/database
- Glova GJ (2003) A test for interaction between brown trout (Salmo trutta) and inanga (Galaxias maculatus) in an artificial stream. Ecology of Freshwater Fish 12(4): 247–253. https://doi.org/10.1046/j.1600-0633.2003.00019.x
- Gozlan RE, St-Hilaire S, Feist SW, Martin P, Kent ML (2005) Disease threat to European fish. Nature 435(7045): e1046. https://doi.org/10.1038/4351046a
- Guzzo MM, Blanchfield PJ (2017) Climate change alters the quantity and phenology of habitat for lake trout (Salvelinus namaycush) in small Boreal Shield lakes. Canadian Journal of Fisheries and Aquatic Sciences 74(6): 871–884. https://doi.org/10.1139/cjfas-2016-0190
- Guzzo MM, Blanchfield PJ, Rennie MD (2017) Behavioral responses to annual temperature variation alter the dominant energy pathway, growth, and condition of a cold-water predator. Proceedings of the National Academy of Sciences 114(37): 9912–9917. https://doi.org/10.1073/pnas.1702584114
- Hardy RW (2002) Rainbow trout, Oncorhynchus mykiss. In: Webster CD, Lim C (Eds) Nutrient requirements and feeding of finfish for aquaculture. CAB International, Oxon, 184–202. https://doi.org/10.1079/9780851995199.0184
- Hasegawa K (2020) Invasions of rainbow trout and brown trout in Japan: A comparison of invasiveness and impact on native species. Ecology of Freshwater Fish 29(3): 419–428. https://doi.org/10.1111/eff.12534
- Hewitt GM (2011) Mediterranean peninsulas: the evolution of hotspots. In: Zachos F, Habel J (Eds) Biodiversity hotspots. Springer, Berlin, Heidelberg, 123–147. https://doi.org/10.1007/978-3-642-20992-5_7
- Hisar O, Yanik T, Aras-Hisar S (2003) Hatchery and growth performance of two trout pure breeds, Salvelinus alpinus and Salmo trutta fario, and their hybrid. Israeli Journal of Aquaculture - Bamidgeh 55: 154–159. https://doi.org/10.46989/001c.20357
- Hosmer Jr DW, Lemeshow S, Sturdivant RX (2013) Applied logistic regression. 3rd edn. , John Wiley & Sons, UK, 511 pp. https://doi.org/10.1002/9781118548387
- Hughes KA, Pescott OL, Peyton J, Adriaens T, Cottier‐Cook EJ, Key G, Rabitsch W, Tricarico E, Barnes DKA, Baxter N, Belchier M, Blake D, Convey P, Dawson W, Frohlich D, Gardiner LM, González-Moreno P, James R, Malumphy C, Martin S, Martinou AF, Minchin D, Monaco A, Moore N, Morley SA, Ross K, Shanklin J, Turvey K, Vaughan D, Vaux AGC, Werenkraut V, Winfield IJ, Roy HE (2020) Invasive non-native species likely to threaten biodiversity and ecosystems in the Antarctic Peninsula region. Global Change Biology 26(4): 2702–2716. https://doi.org/10.1111/gcb.14938
- Interesova E, Vilizzi L, Copp GH (2020) Risk screening of the potential invasiveness of non-native freshwater fishes in the River Ob basin (West Siberian Plain, Russia). Regional Environmental Change 20(2): 1–10. https://doi.org/10.1007/s10113-020-01644-3
- Jelić D, Špelić I, Žutinić P (2016) Introduced species community over-dominates endemic ichthyofauna of high Lika plateau (Central Croatia) over a 100 year period. Acta Zoologica Academiae Scientiarum Hungaricae 62(2): 191–216. https://doi.org/10.17109/AZH.62.2.191.2016
- Joy MK, Foote KJ, McNie P, Piria M (2019) The decline of New Zealand’s freshwater fish fauna; the influence of land-use. Marine and Freshwater Research 70(1): 114–124. https://doi.org/10.1071/MF18028
- Juncos R, Beauchamp DA, Vigliano PH (2013) Modeling prey consumption by native and nonnative piscivorous fishes: Implications for competition and impacts on shared prey in an ultraoligotrophic lake in Patagonia. Transactions of the American Fisheries Society 142(1): 268–281. https://doi.org/10.1080/00028487.2012.730109
- Kalayci G, Ozturk RC, Capkin E, Altinok I (2018) Genetic and molecular evidence that brown trout Salmo trutta belonging to the Danubian lineage are a single biological species. Journal of Fish Biology 93(5): 792–804. https://doi.org/10.1111/jfb.13777
- Kanjuh T, Marić A, Piria M, Špelić I, Maguire I, Simonović P (2020) Diversity of brown trout, Salmo trutta (Actinopterygii: Salmoniformes: Salmonidae), in the Danube River basin of Croatia revealed by mitochondrial DNA. Acta Ichthyologica et Piscatoria 50(3): 291–300. https://doi.org/10.3750/AIEP/02939
- Kanjuh T, Tomić T, Marić A, Škraba Jurlina D, Nikolić V, Simonović P (2021) Trout Salmo spp. (Salmoniformes: Salmonidae) molecular diversity in streams on the southern slopes of the Stara Planina Mts. in Serbia. Acta Zoologica Bulgarica 73: 425–429. http://acta-zoologica-bulgarica.eu/2021/002487.pdf
- Kapetanović D, Vardić I, Valić D, Teskeredžić E (2010) Furunculosis in cultured Arctic charr (Salvelinus alpinus) in Croatia. Aquaculture Research 41: e719–e721. https://doi.org/10.1111/j.1365-2109.2010.02589.x
- Karjalainen J, Keskinen T, Pulkkanen M, Marjomaki TJ (2015) Climate change alters the egg development dynamics in cold-water adapted coregonids. Environmental Biology of Fishes 98(4): 979–991. https://doi.org/10.1007/s10641-014-0331-y
- Karjalainen J, Jokinen L, Keskinen T, Marjomaki TJ (2016) Environmental and genetic effects on larval hatching time in two coregonids. Hydrobiologia 780(1): 135–143. https://doi.org/10.1007/s10750-016-2807-6
- Karleuša B, Rubinić J, Radišić M, Krvavica N (2018) Analysis of climate change impact on water supply in Northern Istria (Croatia). Technical Gazette 25(Suppl. 2): 366–374. https://doi.org/10.17559/TV-20170809140304
- Koutsikos N, Zogaris S, Vardakas L, Tachos V, Kalogianni E, Sanda R, Chatzinikolaou Y, Giakoumi S, Economidis PS, Economou AN (2012) Recent contributions to the distribution of the freshwater ichthyofauna in Greece. Mediterranean Marine Science 13(2): 268–277. https://doi.org/10.12681/mms.308
- Koutsikos N, Vardakas L, Zogaris S, Perdikaris C, Kalantzi OI, Economou AN (2019) Does rainbow trout justify its high rank among alien invasive species? Insights from a nationwide survey in Greece. Aquatic Conservation 29(3): 409–423. https://doi.org/10.1002/aqc.3025
- Krkošek M, Ford JS, Morton A, Lele S, Myers RA, Lewis MA (2007) Declining wild salmon populations in relation to parasites from farm salmon. Science 318(5857): 1772–1773. https://doi.org/10.1126/science.1148744
- Latiu C, Cocan D, Uiuiu P, Ihut A, Nicula SA, Constantinescu R, Mireșan V (2020) The Black Sea trout, Salmo labrax Pallas, 1814 (Pisces: Salmonidae) in Romanian waters. Bulletin of University of Agricultural Sciences and Veterinary Medicine Cluj-Napoca. Animal Science and Biotechnologies 77(2): 9–19. https://doi.org/10.15835/buasvmcn-asb:2020.0017
- Leitwein M, Garza JC, Pearse DE (2017) Ancestry and adaptive evolution of anadromous, resident, and adfluvial rainbow trout (Oncorhynchus mykiss) in the San Francisco bay area: Application of adaptive genomic variation to conservation in a highly impacted landscape. Evolutionary Applications 10(1): 56–67. https://doi.org/10.1111/eva.12416
- Lenhardt M, Marković G, Hegediš A, Maletin S, Ćirković M, Marković Z (2011) Non-native and translocated fish species in Serbia and their impact on the native ichthyofauna. Reviews in Fish Biology and Fisheries 21(3): 407–421. https://doi.org/10.1007/s11160-010-9180-8
- Little AG, Loughland I, Seebacher F (2020) What do warming waters mean for fish physiology and fisheries? Journal of Fish Biology 97(2): 328–340. https://doi.org/10.1111/jfb.14402
- MacCrimmon HR (1971) World distribution of rainbow trout (Salmo gairdneri). Journal of the Fisheries Board of Canada 28(5): 663–704. https://doi.org/10.1139/f71-098
- Makhrov AA, Lajus DL (2018) Postglacial colonization of the North European seas by Pacific fishes and lamprey. Contemporary Problems of Ecology 11(3): 247–258. https://doi.org/10.1134/S1995425518030071
- McIntosh AR, Townsend CR (1995) Contrasting predation risks presented by introduced brown trout and native common river galaxias in New Zealand streams. Canadian Journal of Fisheries and Aquatic Sciences 52(9): 1821–1833. https://doi.org/10.1139/f95-175
- McIntosh AR, Crowl TA, Townsend CR (1994) Size-related impacts of introduced brown trout on the distribution of native common river galaxias. New Zealand Journal of Marine and Freshwater Research 28(2): 135–144. https://doi.org/10.1080/00288330.1994.9516602
- Mihinjač T, Sučić I, Špelić I, Vucić M, Ješovnik A (2019) Non-native freshwater fish species in Croatia. Ministarstvo okoliša i energetike Udruga Hyla, 102 pp. [in Croatian]
- Ministry of Agriculture (2020) Implementation plan of the aquaculture sector transformation strategy 2020–2030. https://poljoprivreda.gov.hr/UserDocsImages/dokumenti/novosti/Nacrt_strategije_razvoja_akvakulture_2020_2030_.pdf [in Croatian]
- Moghaddas SD, Abdoli A, Kiabi BH, Rahmani H, Vilizzi L, Copp GH (2021) Identifying invasive fish species threats to RAMSAR wetland sites in the Caspian Sea region—A case study of the Anzali Wetland Complex (Iran). Fisheries Management and Ecology 28(1): 28–39. https://doi.org/10.1111/fme.12453
- Morbey Ye, Addison P, Shuter BJ, Vascotto K (2006) Within-population heterogeneity of habitat use by lake trout Salvelinus namaycush. Journal of Fish Biology 69(6): 1675–1696. https://doi.org/10.1111/j.1095-8649.2006.01236.x
- Morgan IJ, McDonald DG, Wood CM (2001) The cost of living for freshwater fish in a warmer, more polluted world. Global Change Biology 7(4): 345–355. https://doi.org/10.1046/j.1365-2486.2001.00424.x
- Mrdak D, Nikolić V, Tošić A, Simonović P (2012) Molecular and ecological features of the soft-muzzled trout Salmo obtusirostris (Heckel, 1852) in the Zeta River, Montenegro. Biologia 67(1): 222–233. https://doi.org/10.2478/s11756-011-0150-y
- Mršić V (1935) Experiences with domestication of rainbow trout in Yugoslavia. Ribarski vijesnik 35: 5–7. [in Croatian]
- Muhamedagić S, Habibović E (2013) The state and perspective of Danube huchen (Hucho hucho) in Bosnia and Herzegovina. Archives of Polish Fisheries 22(3): 155–160. https://doi.org/10.2478/aopf-2013-0012
- Muir AM, Vecsei P, Pratt TC, Krueger CC, Power M, Reist JD (2013) Ontogenetic shifts in morphology and resource use of cisco Coregonus artedi. Journal of Fish Biology 82(2): 600–617. https://doi.org/10.1111/jfb.12016
- Official Gazette (2005) Fisheries Law [Serbia]. http://demo.paragraf.rs/WebParagrafDemo/ZAKON-O-RIBARSTVU-Sl.-glasnik-RS,-br.-35-94,-38-94-ispr.-i-101-2005-djr.-zakon.htm
- Official Gazette (2018) The law of protection and sustainable use of fish fund. https://www.pravno-informacioni-sistem.rs/SlGlasnikPortal/eli/rep/sgrs/skupstina/zakon/2014/128/2/reg [Serbian]
- Oikonomou A, Leprieur F, Leonardos ID (2014) Biogeography of freshwater fishes of the Balkan Peninsula. Hydrobiologia 738(1): 205–220. https://doi.org/10.1007/s10750-014-1930-5
- Orizaola G, Braña F (2006) Effect of salmonid introduction and other environmental characteristics on amphibian distribution and abundance in mountain lakes of northern Spain. Animal Conservation 9(2): 171–178. https://doi.org/10.1111/j.1469-1795.2006.00023.x
- Peters L, Spatharis S, Dario MA, Dwyer T, Roca IJ, Kintner A, Kanstad-Hanssen Ø, Llewellyn MS, Praebel K (2018) Environmental DNA: A new low-cost monitoring tool for pathogens in salmonid aquaculture. Frontiers in Microbiology 9: e3009. https://doi.org/10.3389/fmicb.2018.03009
- Pinter K, Epifanio J, Unfer G (2019) Release of hatchery-reared brown trout (Salmo trutta) as a threat to wild populations? A case study from Austria. Fisheries Research 219: e105296. https://doi.org/10.1016/j.fishres.2019.05.013
- Piria M, Povž M, Vilizzi L, Zanella D, Simonović P, Copp GH (2016) Risk screening of non‐ native freshwater fishes in Croatia and Slovenia using the Fish Invasiveness Screening Kit. Fisheries Management and Ecology 23(1): 21–31. https://doi.org/10.1111/fme.12147
- Piria M, Copp GH, Dick JT, Duplić A, Groom Q, Jelić D, Lucy FE, Roy HE, Sarat E, Simonović P, Tomljanović T, Tricarico E, Weinlander M, Adámek Z, Bedolfe S, Coughlan NE, Davis E, Dobrzycka-Krahel A, Grgić Z, Kırankaya ŞG, Ekmekçi FG, Lajtner J, Lukas JAY, Koutsikos N, Mennen GJ, Mitić B, Pastorino P, Ruokonen TJ, Skóra ME, Smith ERC, Šprem N, Tarkan AS, Treer T, Vardakas L, Vehanen T, Vilizzi L, Zanella D, Caffrey JM (2017) Tackling invasive alien species in Europe II: Threats and opportunities until 2020. Management of Biological Invasions 8(3): 273–286. https://doi.org/10.3391/mbi.2017.8.3.02
- Piria M, Simonović P, Kalogianni E, Vardakas L, Koutsikos N, Zanella D, Ristovska M, Apostolou A, Adrović A, Mrdak D, Tarkan AS, Milošević D, Zanella LN, Bakiu R, Ekmekçi FG, Povž M, Korro K, Nikolić V, Škrijelj R, Kostov V, Gregori A, Joy MK (2018) Alien freshwater fish species in the Balkans – Vectors and pathways of introduction. Fish and Fisheries 19(1): 138–169. https://doi.org/10.1111/faf.12242
- Piria M, Špelić I, Rezić A, Šprem N (2020) Morphological traits and condition of brown trout Salmo trutta from Žumberak and Samobor mountain streams. Journal of Central European Agriculture 21: 231–245. https://doi.org/10.5513/JCEA01/21.2.2460
- Piria M, Stroil BK, Giannetto D, Tarkan AS, Gavrilović A, Špelić I, Radočaj T, Killi N, Filiz H, Uysal TU, Aldemir C, Kamberi E, Hala E, Bakiu R, Kolitari J, Buda E, Durmishaj Bakiu S, Sadiku E, Bakrač A, Mujić E, Avdić S, Doumpas N, Giovos I, Dinoshi I, Ušanović L, Kalajdžić A, Pešić A, Ćetković I, Marković O, Milošević D, Mrdak D, Sarà G, Bosch Belmar M, Marchessaux G, Trajanovski S, Zdraveski K (2021a) An assessment of regulation, education practices and socio-economic perceptions of non-native aquatic species in the Balkans. Journal of Vertebrate Biology 70(4): e21047. https://doi.org/10.25225/jvb.21047
- Piria M, Radočaj T, Špelić I, Vilizzi L (2021b) The truth behind the fish diversity of the Lika River and its tributaries: are all management efforts worth it? In: 4th Croatian symposium on invasive species with International Participation, Book of Abstracts, 29–30 November 2021, Zagreb, Croatia, 1–38.
- Plumb JM, Blanchfield PJ (2009) Performance of temperature and dissolved oxygen criteria to predict habitat use by lake trout (Salvelinus namaycush). Canadian Journal of Fisheries and Aquatic Sciences 66(11): 2011–2023. https://doi.org/10.1139/F09-129
- Plumb JM, Blanchfield PJ, Abrahams MV (2014) A dynamic-bioenergetics model to assess depth selection and reproductive growth by lake trout (Salvelinus namaycush). Oecologia 175(2): 549–563. https://doi.org/10.1007/s00442-014-2934-6
- Pofuk M (2021) Non-Indigenous parasites of fish in inland waters of Croatia. Croatian Journal of Fisheries: Ribarstvo 79(4): 187–204. https://doi.org/10.2478/cjf-2021-0020
- Pofuk M, Zanella D, Piria M (2017) An overview of the translocated native and non-native fish species in Croatia: pathways, impacts and management. Management of Biological invasions 8: 425–435. https://doi.org/10.3391/mbi.2017.8.3.16
- Pyšek P, Hulme PE, Simberloff D, Bacher S, Blackburn TM, Carlton JT, Dawson W, Essl F, Foxcroft LC, Genovesi P, Jeschke JM, Kühn I, Liebhold AM, Mandrak NE, Meyerson LA, Pauchard A, Pergl J, Roy HE, Seebens H, van Kleunen M, Vilà M, Wingfield MJ, Richardson DM (2020) Scientists’ warning on invasive alien species. Biological Reviews of the Cambridge Philosophical Society 95(6): 1511–1534. https://doi.org/10.1111/brv.12627
- R Core Team (2021) R: A language and environment for statistical computing. Vienna,Austria: R Foundation for Statistical Computing. https://www.r-project.org/
- Radočaj T, Špelić I, Vilizzi L, Povž M, Piria M (2021) Identifying threats from introduced and translocated non-native freshwater fishes in Croatia and Slovenia under current and future climatic conditions. Global Ecology and Conservation 27: e01520. https://doi.org/10.1016/j.gecco.2021.e01520
- Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez J-C, Müller M (2011) pROC: An open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics 12(1): e77. https://doi.org/10.1186/1471-2105-12-77
- Rosenberg AA (2008) The price of lice. Nature 451(7174): 23–24. https://doi.org/10.1038/451023a
- Rubel F, Brugger K, Haslinger K, Auer I (2017) The climate of the European Alps: Shift of very high resolution Köppen-Geiger climate zones 1800–2100. Meteorologische Zeitschrift (Berlin) 26(2): 115–125. https://doi.org/10.1127/metz/2016/0816
- Schindler DW (2001) The cumulative effects of climate warming and other human stresses on Canadian freshwaters in the new millennium. Canadian Journal of Fisheries and Aquatic Sciences 58(1): 18–29. https://doi.org/10.1139/f00-179
- Simonović P, Tošić A, Vassilev M, Apostolou A, Mrdak D, Ristovska M, Kostov V, Nikolić V, Škraba D, Vilizzi L, Copp GH (2013) Risk identification of non-native freshwater fishes in four countries of the Balkans Region using FISK. Mediterranean Marine Science 14: 369–376. https://doi.org/10.12681/mms.337
- Simonović P, Tošić A, Škraba D, Mrdak D, Grujić S, Nikolić V (2014) Effects of stocking with brood fish to manage resident stream dwelling brown trout Salmo cf. trutta L. stock. Journal of Fisheries Sciences 8: 139–152. https://doi.org/10.3153/jfscom.201418
- Simonović P, Vidović Z, Tošić A, Škraba D, Čanak-Atlagić J, Nikolić V (2015) Risks to stocks of native trout of the genus Salmo (Actinopterygii: Salmoniformes: Salmonidae) of Serbia and management for their recovery. Acta Ichthyologica et Piscatoria 45(2): 161–173. https://doi.org/10.3750/AIP2015.45.2.06
- Simonović P, Tošić A, Škraba Jurlina D, Nikolić V, Piria M, Tomljanović T, Šprem N, Mrdak D, Milošević D, Bećiraj A, Dekić R, Povž M (2017) Diversity of brown trout Salmo cf. trutta in the River Danube basin of western Balkans as assessed from the structure of their mitochondrial control region haplotypes. Journal of Ichthyology 57(4): 603–616. https://doi.org/10.1134/S0032945217040154
- Skelton PH (1987) South African Red Data Book. Fishes. South African National Scientific Programmes Report 137. Council for Scientific and Industrial Research, Pretoria.
- Škraba D, Bećiraj A, Šarić I, Ićanović I, Džaferović A, Piria M, Dekić R, Tošić A, Nikolić V, Simonović P (2017) Genotypization of brown trout (Salmo trutta L.) populations from River Una drainage area in Bosnia and Herzegovina and implications for conservation and fishery management. Acta Zoologica Bulgarica 69: 25–30.
- Škraba Jurlina D, Marić A, Mrdak D, Kanjuh T, Špelić I, Nikolić V, Piria M, Simonović P (2020) Alternative life-history in native trout (Salmo spp.) suppresses the invasive effect of alien trout strains introduced into streams in the western part of the Balkans. Frontiers in Ecology and Evolution 8: e188. https://doi.org/10.3389/fevo.2020.00188
- Spens J, Alanärä A, Eriksson LO (2007) Nonnative brook trout (Salvelinus fontinalis) and the demise of native brown trout (Salmo trutta) in northern boreal lakes: Stealthy, long-term patterns? Canadian Journal of Fisheries and Aquatic Sciences 64(4): 654–664. https://doi.org/10.1139/f07-040
- Stagl J, Hattermann F (2016) Impacts of climate change on riverine ecosystems: Alterations of ecologically relevant flow dynamics in the Danube River and its major tributaries. Water (Basel) 8(12): e566. https://doi.org/10.3390/w8120566
- Stanković D, Crivelli AJ, Snoj A (2015) Rainbow trout in Europe: Introduction, naturalization, and impacts. Reviews in Fisheries Science & Aquaculture 23(1): 39–71. https://doi.org/10.1080/23308249.2015.1024825
- Stoumboudi MT, Barbieri R, Kalogianni E (2017) First report of an established population of Oncorhynchus mykiss (Walbaum, 1792) (Salmonidae) on the Island of Crete, Greece. Acta Zoologica Bulgarica 9: 99–104.
- Sušnik S, Weiss S, Odak T, Delling B, Treer T, Snoj A (2007) Reticulate evolution: ancient introgression of the Adriatic brown trout mtDNA in softmouth trout Salmo obtusirostris (Teleostei: Salmonidae). Biological Journal of the Linnean Society 90(1): 139–152. https://doi.org/10.1111/j.1095-8312.2007.00717.x
- Tarkan AS, Vilizzi L, Top N, Ekmekçi FG, Stebbing PD, Copp GH (2017) Identification of potentially invasive freshwater fishes, including translocated species, in Turkey using the Aquatic Species Invasiveness Screening Kit (AS‐ISK). International Review of Hydrobiology 102(1–2): 47–56. https://doi.org/10.1002/iroh.201601877
- Tarkan AS, Yoğurtçuoğlu B, Ekmekçi FG, Clarke SA, Wood LE, Vilizzi L, Copp GH (2020) First application in Turkey of the European Non-native Species in Aquaculture Risk Assessment Scheme to evaluate farmed non-native striped catfish Pangasianodon hypophthalmus. Fisheries Management and Ecology 27(2): 123–131. https://doi.org/10.1111/fme.12387
- Tarkan AS, Emiroğlu Ö, Aksu S, Başkurt S, Aksu İ, Vilizzi L, Yoğurtçuoğlu B (2022) Coupling molecular analysis with risk assessment to investigate the origin, distribution and potential impact of non-native species: Application to ruffe Gymnocephalus cernua in Turkey. The European Zoological Journal 89: 102–114. https://doi.org/10.1080/24750263.2021.2022222
- Tošić A, Škraba D, Nikolić V, Čanak Atlagić J, Mrdak D, Simonović P (2016) Haplotype diversity of brown trout in the broader Iron Gate area. Turkish Journal of Zoology 40: 655–662. https://doi.org/10.3906/zoo-1510-54
- Trenberth KE (2011) Changes in precipitation with climate change. Climate Research 47(1): 123–138. https://doi.org/10.3354/cr00953
- Vehanen T, Piria M, Kubečka J, Skov C, Kelly F, Pokki H, Eskelinen P, Rahikainen M, Keskinen T, Artell J, Romakkaniemi A, Suić J, Adámek Z, Heimlich R, Chalupa P, Ženíšková H, Lyach R, Berg S, Birnie-Gauvin K, Jepsen N, Koed A, Ingemann Pedersen M, Rasmussen G, Gargan P, Roche W (2020) Systems and methodologies of data collection in inland fisheries of Europe. FAO Fisheries and Aquaculture Technical Paper 649: 1–168. https://doi.org/10.4060/ca7993en
- Ventura M, Tiberti R, Buchaca T, Bunay D, Sabas I, Miro A (2017) Why Should We Preserve Fishless High Mountain Lakes? In: Catalan J, Ninot JM, Aniz MM (Eds) High mountain conservation in a changing world. Advances in Global Change Research 62: 181–207. https://doi.org/10.1007/978-3-319-55982-7_8
- Vergara IA, Norambuena T, Ferrada E, Slater AW, Melo F (2008) StAR: A simple tool for the statistical comparison of ROC curves. BMC Bioinformatics 9(1): e265. https://doi.org/10.1186/1471-2105-9-265
- Vilà M, Basnou C, Pyšek P, Josefsson M, Genovesi P, Gollasch S, Nentwig W, Olenin S, Roques A, Roy D, Hulme PE (2010) How well do we understand the impacts of alien species on ecosystem services? A pan-European, cross-taxa assessment. Frontiers in Ecology and the Environment 8(3): 135–144. https://doi.org/10.1890/080083
- Vilizzi L, Hill JE, Piria M, Copp GH (2022) A protocol for screening potentially invasive non-native species using Weed Risk Assessment-type decision-support toolkits. Science of the Total Environment 832: e154966. https://doi.org/10.1016/j.scitotenv.2022.154966
- Weiss S, Schenekar T (2016) Genetic evaluation of the self-sustaining status of a population of the endangered Danube salmon, Hucho hucho. Hydrobiologia 775(1): 153–165. https://doi.org/10.1007/s10750-016-2726-6
- Wood LE, Guilder J, Brennan ML, Birland NJ, Taleti V, Stinton N, Taylor NGH, Thrush MA (2021) Biosecurity and the ornamental fish trade: A stakeholder perspective in England. Journal of Fish Biology 100(2): 352–365. https://doi.org/10.1111/jfb.14928
- Yoğurtçuoğlu B, Bucak T, Ekmekçi FG, Kaya C, Tarkan AS (2021) Mapping the establishment and invasiveness potential of rainbow trout (Oncorhynchus mykiss) in Turkey: With special emphasis on the conservation of native salmonids. Frontiers in Ecology and Evolution 8: e599881. https://doi.org/10.3389/fevo.2020.599881
- Young KA, Dunham JB, Stephenson JF, Terreau A, Thailly AF, Gajardo G, Garcia de Leaniz C (2010) A trial of two trouts: Comparing the impacts of rainbow and brown trout on a native galaxiid. Animal Conservation 13(4): 399–410. https://doi.org/10.1111/j.1469-1795.2010.00354.x
- Zupančič P, Tisaj D, Lah Lj (2008) Rare and endangered freshwater fishes of Croatia, Slovenia and Bosnia and Herzegovina - Adriatic basin. Dolsko AZV, 79 pp.
Supplementary material
Combined AS-ISK report including the 68 screenings for the 17 salmonid species screened for the Danube and Adriatic basins of Bosnia and Herzegovina, Croatia, Montenegro and Serbia (including Kosovo)
Data type: pdf file
Explanation note: Combined AS-ISK report including the 68 screenings for the 17 salmonid species screened for the Danube and Adriatic basins of Bosnia and Herzegovina, Croatia, Montenegro and Serbia (including Kosovo).